- Record: found
- Abstract: found
- Article: found
Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients
Abstract
Purpose
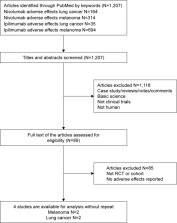
The aim of this study was to evaluate the risk of immune-related adverse events (irAEs) among cancer patients receiving nivolumab-plus-ipilimumab therapy and nivolumab monotherapy.
Patients and methods
PubMed and Web of Science were searched for related studies from inception to June 2018. Eligible studies included randomized controlled trials comparing nivolumab-plus-ipilimumab with nivolumab alone in cancer patients reporting on all-grade (grade 1–4) and high-grade (grade 3/4) irAEs. Paired reviewers selected studies for inclusion and extracted data. The odds risk and 95% CI were calculated.
Results
A total of 2,946 patients from four studies were included in the meta-analysis. The underlying malignancies included lung cancer (two trials) and melanoma (two trials). Compared with nivolumab monotherapy, the nivolumab-plus-ipilimumab therapy was associated with a significantly higher risk of all- and high-grade irAEs such as pruritus, rash, diarrhea, colitis, alanine aminotransferase elevation, and pneumonitis.
Most cited references17
- Record: found
- Abstract: not found
- Article: not found
Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review
- Record: found
- Abstract: found
- Article: not found
CTLA-4 is a second receptor for the B cell activation antigen B7

- Record: found
- Abstract: found
- Article: found