- Record: found
- Abstract: found
- Article: found
Antiapoptotic Effects of EGb 761
Read this article at
Abstract
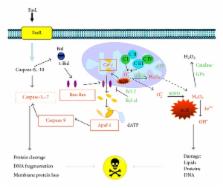
Ginkgo biloba extracts have long been used in Chinese traditional medicine for hundreds of years. The most significant extract obtained from Ginkgo biloba leaves has been EGb 761, a widely used phytopharmaceutical product in Europe. EGb 761 is a well-defined mixture of active compounds, which contains two main active substances: flavonoid glycosides (24–26%) and terpene lactones (6–8%). These compounds have shown antiapoptotic effects through the protection of mitochondrial membrane integrity, inhibition of mitochondrial cytochrome c release, enhancement of antiapoptotic protein transcription, and reduction of caspase transcription and DNA fragmentation. Other effects include the reduction of oxidative stress (which has been related to the occurrence of vascular, degenerative, and proliferative diseases), coupled to strong induction of phase II-detoxifying and cellular defense enzymes by Nrf2/ARE activation, in addition to the modulation of transcription factors, such as CREB, HIF-1 α , NF- κ B, AP-1, and p53, involved in the apoptosis process. This work reviews experimental results about the antiapoptotic effects induced by the standardized extract of Ginkgo biloba leaves (EGb 761).
Related collections
Most cited references171
- Record: found
- Abstract: found
- Article: not found
Caspases: the executioners of apoptosis.
- Record: found
- Abstract: found
- Article: not found
Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases.
- Record: found
- Abstract: found
- Article: not found