- Record: found
- Abstract: found
- Article: found
CSF A β42/A β40 and A β42/A β38 ratios: better diagnostic markers of Alzheimer disease
Read this article at
Abstract
Objective
The diagnostic accuracy of cerebrospinal fluid ( CSF) biomarkers for Alzheimer's disease ( AD) must be improved before widespread clinical use. This study aimed to determine whether CSF A β42/A β40 and A β42/A β38 ratios are better diagnostic biomarkers of AD during both predementia and dementia stages in comparison to CSF A β42 alone.
Methods
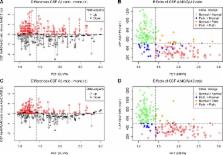
The study comprised three different cohorts ( n = 1182) in whom CSF levels of A β42, A β40, and A β38 were assessed. CSF A βs were quantified using three different immunoassays (Euroimmun, Meso Scale Discovery, Quanterix). As reference standard, we used either amyloid ( 18F‐flutemetamol) positron emission tomography ( PET) imaging ( n = 215) or clinical diagnosis ( n = 967) of well‐characterized patients.
Results
When using three different immunoassays in cases with subjective cognitive decline and mild cognitive impairment, the CSF A β42/A β40 and A β42/A β38 ratios were significantly better predictors of abnormal amyloid PET than CSF A β42. Lower A β42, A β42/A β40, and A β42/A β38 ratios, but not A β40 and A β38, correlated with smaller hippocampal volumes measured by magnetic resonance imaging. However, lower A β38, A β40, and A β42, but not the ratios, correlated with non‐ AD‐specific subcortical changes, that is, larger lateral ventricles and white matter lesions. Further, the A β42/A β40 and A β42/A β38 ratios showed increased accuracy compared to A β42 when distinguishing AD from dementia with Lewy bodies or Parkinson's disease dementia and subcortical vascular dementia, where all A βs (including A β42) were decreased.
Interpretation
The CSF A β42/A β40 and A β42/A β38 ratios are significantly better than CSF A β42 to detect brain amyloid deposition in prodromal AD and to differentiate AD dementia from non‐ AD dementias. The ratios reflect AD‐type pathology better, whereas decline in CSF A β42 is also associated with non‐AD subcortical pathologies. These findings strongly suggest that the ratios rather than CSF A β42 should be used in the clinical work‐up of AD.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
APP processing and synaptic function.
- Record: found
- Abstract: found
- Article: not found
Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43).
- Record: found
- Abstract: found
- Article: not found