- Record: found
- Abstract: found
- Article: found
Growth Differentiation Factor 15 Regulates Oxidative Stress-Dependent Ferroptosis Post Spinal Cord Injury by Stabilizing the p62-Keap1-Nrf2 Signaling Pathway
Read this article at
Abstract
Background
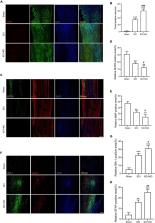
Spinal cord injury (SCI) is a severe traumatic disorder of the central nervous system (CNS) that causes irreversible damage to the nervous tissue. The consequent hemorrhage contributed by trauma induces neuronal ferroptosis post SCI, which is an important death mode to mediate neuronal loss. Growth differentiation factor 15 (GDF15) is a cytokine that regulates cell proliferation, differentiation, and death. However, the specific role of GDF15 in neuronal ferroptosis post SCI remains unknown.
Materials and Methods
Neuronal ferroptosis in vitro was measured by detection of lipid peroxidation, glutathione, iron content, and reactive oxidative stress. In vivo, western blotting and immunofluorescence (IF) staining was utilized to measure ferroptosis post SCI. IF staining, TUNEL staining, hematoxylin-eosin staining, and Nissl staining were used to measure neurological damage. Finally, locomotor function recovery was analyzed using the Basso Mouse Scale and Louisville Swim Scale.
Results
GDF15 was significantly increased in neuronal ferroptosis and silencing GDF15 aggravated ferroptosis both in vitro and in vivo. Besides, GDF15-mediated inhibition of neuronal ferroptosis is through p62-dependent Keap1-Nrf2 pathway. In SCI mice, knockdown of GDF15 significantly exacerbated neuronal death, interfered with axon regeneration and remyelination, aggravated ferroptosis-mediated neuroinflammation, and restrained locomotor recovery.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: Death by Lipid Peroxidation.
- Record: found
- Abstract: found
- Article: not found