- Record: found
- Abstract: found
- Article: found
Mutual induced fit transition structure stabilization of corannulene's bowl-to-bowl inversion in a perylene bisimide cyclophane†
Read this article at
Abstract
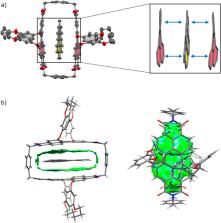
Corannulene is known to undergo a fast bowl-to-bowl inversion at r.t. via a planar transition structure (TS). Herein we present the catalysis of this process within a perylene bisimide (PBI) cyclophane composed of chirally twisted, non-planar chromophores, linked by para-xylylene spacers. Variable temperature NMR studies reveal that the bowl-to-bowl inversion is significantly accelerated within the cyclophane template despite the structural non-complementarity between the binding site of the host and the TS of the guest. The observed acceleration corresponds to a decrease in the bowl-to-bowl inversion barrier of 11.6 kJ mol −1 compared to the uncatalyzed process. Comparative binding studies for corannulene (20 π-electrons) and other planar polycyclic aromatic hydrocarbons (PAHs) with 14 to 24 π-electrons were applied to rationalize this barrier reduction. They revealed high binding constants that reach, in tetrachloromethane as a solvent, the picomolar range for the largest guest coronene. Computational models corroborate these experimental results and suggest that both TS stabilization and ground state destabilization contribute to the observed catalytic effect. Hereby, we find a “mutual induced fit” between host and guest in the TS complex, such that mutual geometric adaptation of the energetically favored planar TS and curved π-systems of the host results in an unprecedented non-planar TS of corannulene. Concomitant partial planarization of the PBI units optimizes noncovalent TS stabilization by π–π stacking interactions. This observation of a “mutual induced fit” in the TS of a host–guest complex was further validated experimentally by single crystal X-ray analysis of a host–guest complex with coronene as a qualitative transition state analogue.
Abstract
Combined experimental and computational studies find a perylene bisimide cyclophane to catalyse the corannulene bowl-inversion. Host and guest undergo mutual geometric adaptation, maximizing transition structure stabilization from π–π stacking.

Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found