- Record: found
- Abstract: found
- Article: found
The immunology of hypertension
Read this article at
Abstract
Emerging evidence indicates that both innate and adaptive immunity contribute to hypertension. Efforts to understand mechanisms of immune activation in hypertension are defining not only new mechanisms of disease but also new therapeutic options for its treatment.
Abstract
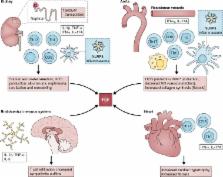
Although systemic hypertension affects a large proportion of the population, its etiology remains poorly defined. Emerging evidence supports the concept that immune cells become activated and enter target organs, including the vasculature and the kidney, in this disease. Mediators released by these cells, including reactive oxygen species, metalloproteinases, cytokines, and antibodies promote dysfunction of the target organs and cause damage. In vessels, these factors enhance constriction, remodeling, and rarefaction. In the kidney, these mediators increase expression and activation of sodium transporters, and cause interstitial fibrosis and glomerular injury. Factors common to hypertension, including oxidative stress, increased interstitial sodium, cytokine production, and inflammasome activation promote immune activation in hypertension. Recent data suggest that isolevuglandin-modified self-proteins in antigen-presenting cells are immunogenic, promoting cytokine production by the cells in which they are formed and T cell activation. Efforts to prevent and reverse immune activation may prove beneficial in preventing the long-term sequelae of hypertension and its related cardiovascular diseases.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Inflammation, immunity, and hypertensive end-organ damage.
- Record: found
- Abstract: found
- Article: not found
Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction.
- Record: found
- Abstract: found
- Article: not found