- Record: found
- Abstract: found
- Article: found
Molecular Mechanisms of Dermal Aging and Antiaging Approaches
Read this article at
Abstract
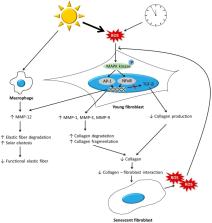
The dermis is primarily composed of the extracellular matrix (ECM) and fibroblasts. During the aging process, the dermis undergoes significant changes. Collagen, which is a major component of ECM, becomes fragmented and coarsely distributed, and its total amount decreases. This is mainly due to increased activity of matrix metalloproteinases, and impaired transforming growth factor-β signaling induced by reactive oxygen species generated during aging. The reduction in the amount of collagen hinders the mechanical interaction between fibroblasts and the ECM, and consequently leads to the deterioration of fibroblast function and further decrease in the amount of dermal collagen. Other ECM components, including elastic fibers, glycosaminglycans (GAGs), and proteoglycans (PGs), also change during aging, ultimately leading to a reduction in the amount of functional components. Elastic fibers decrease in intrinsically aged skin, but accumulate abnormally in photoaged skin. The changes in the levels of GAGs and PGs are highly diverse, and previous studies have reported conflicting results. A reduction in the levels of functional dermal components results in the emergence of clinical aging features, such as wrinkles and reduced elasticity. Various antiaging approaches, including topicals, energy-based procedures, and dermal fillers, can restore the molecular features of dermal aging with clinical efficacy. This review summarizes the current understanding of skin aging at the molecular level, and associated treatments, to put some of the new antiaging technology that has emerged in this rapidly expanding field into molecular context.
Related collections
Most cited references141
- Record: found
- Abstract: found
- Article: not found
Structure and function of matrix metalloproteinases and TIMPs.
- Record: found
- Abstract: found
- Article: not found
Pathophysiology of premature skin aging induced by ultraviolet light.
- Record: found
- Abstract: found
- Article: not found