- Record: found
- Abstract: found
- Article: found
Tissue origin of cytotoxic natural killer cells dictates their differential roles in mouse digit tip regeneration and progenitor cell survival

Read this article at
Summary
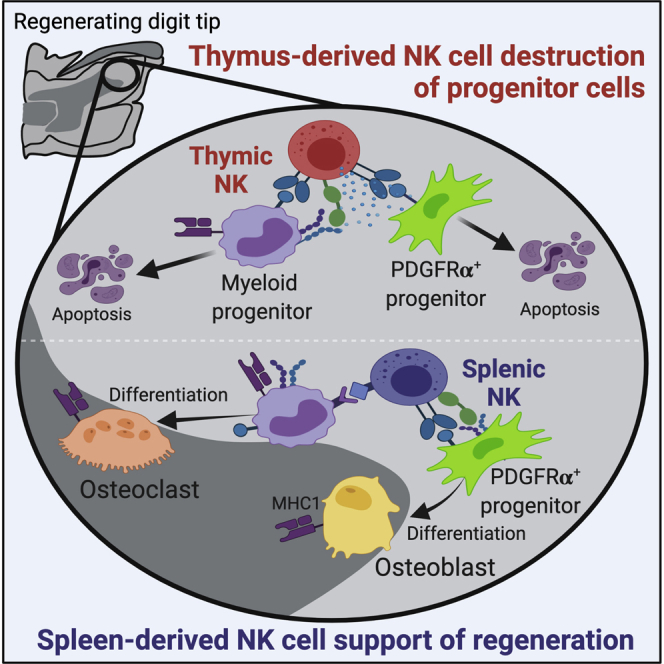
Regeneration of amputated digit tips relies on mesenchymal progenitor cells and their differentiation into replacement bone and tissue stroma. Natural killer (NK) cells have well-characterized roles in antigen-independent killing of virally infected, pre-tumorous, or stressed cells; however, the potential for cytotoxic activity against regenerative progenitor cells is unclear. We identified NK cell recruitment to the regenerating digit tip, and NK cytotoxicity was observed against osteoclast and osteoblast progenitors. Adoptive cell transplants of spleen NK (SpNK) or thymus NK (ThNK) donor cells into immunodeficient mice demonstrated ThNK cell-induced apoptosis with a reduction in osteoclasts, osteoblasts, and proliferative cells, resulting in inhibition of regeneration. Adoptive transfer of NK cells deficient in NK cell activation genes identified that promotion of regeneration by SpNK cells requires Ncr1, whereas inhibition by ThNK cells is mediated via Klrk1 and perforin. Successful future therapies aimed at enhancing regeneration will require a deeper understanding of progenitor cell protection from NK cell cytotoxicity.
Graphical abstract
Highlights
-
•
NK cell ablation delays regeneration, demonstrating a regulatory role in vivo
-
•
Spleen-derived NK (SpNK) and thymus-derived (ThNK) cells are cytotoxic ex vivo
-
•
SpNK cells enhance regeneration in vivo without progenitor cell cytotoxicity
-
•
ThNK cells inhibit regeneration in vivo via progenitor cell destruction
Abstract
Dastagir et al. identified two NK cell subsets with divergent activity on osteoclast and osteoblast progenitor cells critical for successful digit tip regeneration. Spleen-derived NK cells are non-cytotoxic in vivo and enhance regeneration, but thymus-derived NK cells maintain cytotoxicity and inhibit regeneration. These findings reveal new insights into potential mechanisms of regenerative failure in human tissue.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Functions of natural killer cells.
- Record: found
- Abstract: found
- Article: not found
Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA.

- Record: found
- Abstract: found
- Article: found
Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.