- Record: found
- Abstract: found
- Article: found
Potential Role of Ocular Microbiome, Host Genotype, Tear Cytokines, and Environmental Factors in Corneal Infiltrative Events in Contact Lens Wearers
Read this article at
Abstract
Purpose
The purpose of this study was to explore differences in genotype, ocular surface microbiome, tear inflammatory markers, and environmental and behavioral exposures in soft contact lens (SCL) wearers with and without a history of corneal infiltrative events (CIEs).
Methods
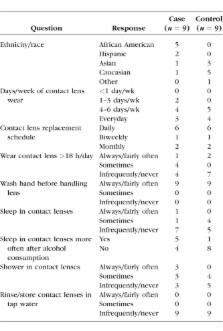
Nine SCL wearers with a recent CIE and nine age-, sex-, and SCL material- and modality-matched controls were enrolled. The Contact Lens Risk Survey, slit-lamp examination data, basal tears, conjunctival microbial cultures, and peripheral blood samples were collected. Tear inflammatory mediator concentrations, genomic DNA from swabs, and whole exome sequencing of blood samples were quantified.
Results
There were no marked differences in SCL wear behaviors or exposures between case and control subjects. Predominant organisms detected among case and control subjects were Staphylococcus, Propionibacterium, Streptococcus, and Corynebacterium. Marginally higher levels of Neisseria were found in three of nine cases but zero of nine control samples ( P = 0.056). A potentially deleterious missense single nucleotide polymorphism (SNP) variant in IL-6 Signal Transducer ( IL6ST) was found in seven of eight cases and zero of nine controls (rs2228046; P = 0.03). The concentration of tear IL-6 was significantly higher in cases (4.5 [range, 2.1 to 6.2] pg/mL) versus controls (3.5 [range, 2.5 to 6.6] Pg/mL; = 0.02).
Conclusions
Tear IL-6 concentration was higher, and SNP variants were detected in subjects with a history of CIEs compared with healthy controls. The synthesis, signaling, and ocular surface cytokine concentration of IL-6 may be related to susceptibility to CIE. A larger study population is required to further explore relationships between genetic variations, the ocular surface microbiome, inflammatory mediators, and environmental exposures.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Interleukin-6 and Diabetes: The Good, the Bad, or the Indifferent?
- Record: found
- Abstract: found
- Article: not found
Diversity of bacteria at healthy human conjunctiva.
- Record: found
- Abstract: found
- Article: not found