- Record: found
- Abstract: found
- Article: found
Non-Pulmonary Vein Triggers of Atrial Fibrillation Are Likely to Arise from Low-Voltage Areas in the Left Atrium
Read this article at
Abstract
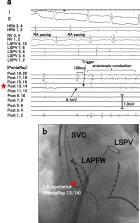
The pathophysiology of non-pulmonary vein (PV) triggers of atrial fibrillation (AF) is unclear. We hypothesized that left atrial non-PV (LANPV) triggers are associated with atrial tissue degeneration. This study analyzed 431 patients that underwent catheter ablation (mean age 62 yrs, 303 men, 255 paroxysmal AF [pAF] patients). Clinical and electrophysiological characteristics of non-PV trigger were analyzed. Fifty non-PV triggers in 40 patients (9.3%) were documented; LANPV triggers were the most prevalent (n = 19, 38%). LANPV triggers were correlated with non-paroxysmal AF (non-pAF) (OR 3.31, p = 0.04) whereas right atrial non-PV (RANPV) triggers (n = 14) and SVC triggers (n = 17) were not. The voltage at the LANPV sites during SR was 0.3 ± 0.16 mV (p < 0.001 vs. control site). Low-voltage areas (LVAs) in the LA were significantly greater in non-pAF compared to pAF (14.2% vs. 5.8%, p < 0.01). RANPV trigger sites had preserved voltage (0.74 ± 0.48 mV). Long-term outcomes of patients with non-PV triggers treated with tailored targeting strategies were not significantly inferior to those without non-PV triggers. In conclusion, non-PV triggers arise from the LA with degeneration, which may have an important role in AF persistence. A trigger-oriented, patient-tailored ablation strategy considering LA voltage map may be feasible and effective in persistent/recurrent AF.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation.
- Record: found
- Abstract: found
- Article: not found
Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy.
- Record: found
- Abstract: found
- Article: not found