- Record: found
- Abstract: found
- Article: found
Role of extracellular vesicles from adipose tissue‐ and bone marrow‐mesenchymal stromal cells in endothelial proliferation and chondrogenesis
Read this article at
Abstract
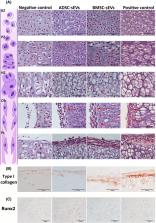
The secretome of mesenchymal stromal cells (MSCs) derived from different tissue sources is considered an innovative therapeutic tool for regenerative medicine. Although adipose tissue‐and bone marrow‐derived MSCs (ADSCs and BMSCs, respectively) share many biological features, the different tissue origins can be mirrored by variations in their secretory profile, and in particular in the secreted extracellular vesicles (EVs). In this study, we carried out a detailed and comparative characterization of middle‐ and small‐sized EVs (mEVs and sEVs, respectively) released by either ADSCs or BMSCs. Their involvement in an endochondral ossification setting was investigated using ex vivo metatarsal culture models that allowed to explore both blood vessel sprouting and bone growth plate dynamics. Although EVs separated from both cell sources presented similar characteristics in terms of size, concentration, and marker expression, they exhibited different characteristics in terms of protein content and functional effects. ADSC‐EVs overexpressed pro‐angiogenic factors in comparison to the BMSC‐counterpart, and, consequently, they were able to induce a significant increase in endothelial cord outgrowth. On the other hand, BMSC‐EVs contained a higher amount of pro‐differentiation and chemotactic proteins, and they were able to prompt growth plate organization. The present study highlights the importance of selecting the appropriate cell source of EVs for targeted therapeutic applications.
Abstract
This study shows how the differences among bone marrow‐ and adipose tissue‐derived mesenchymal stromal cells (BMSCs and ADSCs, respectively) are mirrored in the corresponding extracellular vesicles (EVs). The role of ADSCs‐ and BMSCs‐derived EVs in an endochondral ossification setting was investigated using ex vivo metatarsal culture models that allowed to explore both blood vessel sprouting and bone growth plate dynamics.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement.

- Record: found
- Abstract: found
- Article: found
