- Record: found
- Abstract: found
- Article: found
Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): an indicator of intestinal permeability in broiler chickens
Read this article at
Abstract
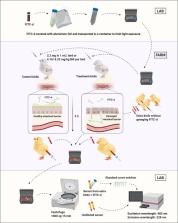
Fluorescein isothiocyanate-dextran ( FITC-d) is being used as an indicator of intestinal paracellular permeability in poultry research. Especially with the industry moving toward antibiotic-free production, intestinal function and integrity issues have been a research focus. An increasing number of scientific conference abstracts and peer-reviewed journal publications have shown that 4-kDa FITC-d is an efficient marker candidate for measurement of intestinal permeability and can be applied in broiler research. However, experimental protocols vary by personnel, instruments used, and research institution, and potential concerns related to this assay have yet to receive the same amount of attention. Understanding protocol consistency within and across laboratories is vital for obtaining accurate, consistent, and comparable experimental results. This review is aimed to 1) summarize different FITC-d assays in broiler research from peer-reviewed publications during the past 6 yr and 2) discuss factors that can potentially affect intestinal permeability results when conducting the FITC-d assay. In summary, it is essential to pay attention to details, including gavage dose, fasting period, sample handling and lab analysis details when conducting the assay in broiler research. Differences in birds (breed/strain, age, and gender) and experimental design (diet, health status/challenge model, and sampling age) need to be considered when comparing serum FITC-d concentration results between different in vivo animal trials.
Related collections
Most cited references60

- Record: found
- Abstract: found
- Article: found
Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability
- Record: found
- Abstract: found
- Article: not found
The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system.
- Record: found
- Abstract: found
- Article: not found