- Record: found
- Abstract: found
- Article: not found
Physiology and pathophysiology of matrix metalloproteases
Read this article at
Abstract
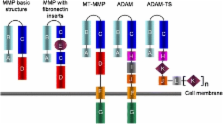
Matrix metalloproteases (MMPs) comprise a family of enzymes that cleave protein substrates based on a conserved mechanism involving activation of an active site-bound water molecule by a Zn 2+ ion. Although the catalytic domain of MMPs is structurally highly similar, there are many differences with respect to substrate specificity, cellular and tissue localization, membrane binding and regulation that make this a very versatile family of enzymes with a multitude of physiological functions, many of which are still not fully understood. Essentially, all members of the MMP family have been linked to disease development, notably to cancer metastasis, chronic inflammation and the ensuing tissue damage as well as to neurological disorders. This has stimulated a flurry of studies into MMP inhibitors as therapeutic agents, as well as into measuring MMP levels as diagnostic or prognostic markers. As with most protein families, deciphering the function(s) of MMPs is difficult, as they can modify many proteins. Which of these reactions are physiologically or pathophysiologically relevant is often not clear, although studies on knockout animals, human genetic and epigenetic, as well as biochemical studies using natural or synthetic inhibitors have provided insight to a great extent. In this review, we will give an overview of 23 members of the human MMP family and describe functions, linkages to disease and structural and mechanistic features. MMPs can be grouped into soluble (including matrilysins) and membrane-anchored species. We adhere to the ‘MMP nomenclature’ and provide the reader with reference to the many, often diverse, names for this enzyme family in the introduction.
Related collections
Most cited references226
- Record: found
- Abstract: found
- Article: not found
How matrix metalloproteinases regulate cell behavior.
- Record: found
- Abstract: not found
- Article: not found
