- Record: found
- Abstract: found
- Article: found
Expression and function of proton-sensing G-protein-coupled receptors in inflammatory pain
Read this article at
Abstract
Background
Chronic inflammatory pain, when not effectively treated, is a costly health problem and has a harmful effect on all aspects of health-related quality of life. Despite the availability of pharmacologic treatments, chronic inflammatory pain remains inadequately treated. Understanding the nociceptive signaling pathways of such pain is therefore important in developing long-acting treatments with limited side effects. High local proton concentrations (tissue acidosis) causing direct excitation or modulation of nociceptive sensory neurons by proton-sensing receptors are responsible for pain in some inflammatory pain conditions. We previously found that all four proton-sensing G-protein-coupled receptors (GPCRs) are expressed in pain-relevant loci (dorsal root ganglia, DRG), which suggests their possible involvement in nociception, but their functions in pain remain unclear.
Results
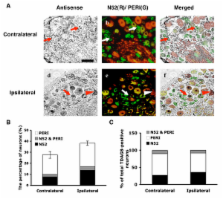
In this study, we first demonstrated differential change in expression of proton-sensing GPCRs in peripheral inflammation induced by the inflammatory agents capsaicin, carrageenan, and complete Freund's adjuvant (CFA). In particular, the expression of TDAG8, one proton-sensing GPCR, was increased 24 hours after CFA injection because of increased number of DRG neurons expressing TDAG8. The number of DRG neurons expressing both TDAG8 and transient receptor potential vanilloid 1 (TRPV1) was increased as well. Further studies revealed that TDAG8 activation sensitized the TRPV1 response to capsaicin, suggesting that TDAG8 could be involved in CFA-induced chronic inflammatory pain through regulation of TRPV1 function.
Conclusion
Each subtype of the OGR1 family was expressed differently, which may reflect differences between models in duration and magnitude of hyperalgesia. Given that TDAG8 and TRPV1 expression increased after CFA-induced inflammation and that TDAG8 activation can lead to TRPV1 sensitization, it suggests that high concentrations of protons after inflammation may not only directly activate proton-sensing ion channels (such as TRPV1) to cause pain but also act on proton-sensing GPCRs to regulate the development of hyperalgesia.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Impaired nociception and pain sensation in mice lacking the capsaicin receptor.
- Record: found
- Abstract: found
- Article: not found