- Record: found
- Abstract: found
- Article: found
Potentiating Therapeutic Effects of Epidermal Growth Factor Receptor Inhibition in Triple-Negative Breast Cancer
Read this article at
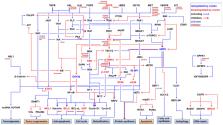
Abstract
Triple-negative breast cancer (TNBC) is a subset of breast cancer with aggressive characteristics and few therapeutic options. The lack of an appropriate therapeutic target is a challenging issue in treating TNBC. Although a high level expression of epidermal growth factor receptor (EGFR) has been associated with a poor prognosis among patients with TNBC, targeted anti-EGFR therapies have demonstrated limited efficacy for TNBC treatment in both clinical and preclinical settings. However, with the advantage of a number of clinically approved EGFR inhibitors (EGFRis), combination strategies have been explored as a promising approach to overcome the intrinsic resistance of TNBC to EGFRis. In this review, we analyzed the literature on the combination of EGFRis with other molecularly targeted therapeutics or conventional chemotherapeutics to understand the current knowledge and to provide potential therapeutic options for TNBC treatment.
Related collections
Most cited references649
- Record: found
- Abstract: found
- Article: not found
The biology, function, and biomedical applications of exosomes

- Record: found
- Abstract: found
- Article: not found
Comprehensive molecular portraits of human breast tumors
- Record: found
- Abstract: found
- Article: not found