- Record: found
- Abstract: found
- Article: found
Dihydroartemisinin: A Potential Drug for the Treatment of Malignancies and Inflammatory Diseases
Read this article at
Abstract
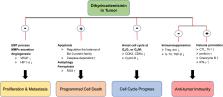
Dihydroartemisinin (DHA) has been globally recognized for its efficacy and safety in the clinical treatment of malaria for decades. Recently, it has been found that DHA inhibits malignant tumor growth and regulates immune system function in addition to anti-malaria. In parasites and tumors, DHA causes severe oxidative stress by inducing excessive reactive oxygen species production. DHA also kills tumor cells by inducing programmed cell death, blocking cell cycle and enhancing anti-tumor immunity. In addition, DHA inhibits inflammation by reducing the inflammatory cells infiltration and suppressing the production of pro-inflammatory cytokines. Further, genomics, proteomics, metabolomics and network pharmacology of DHA therapy provide the basis for elucidating the pharmacological effects of DHA. This review provides a summary of the recent research progress of DHA in anti-tumor, inhibition of inflammatory diseases and the relevant pharmacological mechanisms. With further research of DHA, it is likely that DHA will become an alternative therapy in the clinical treatment of malignant tumors and inflammatory diseases.
Related collections
Most cited references142
- Record: found
- Abstract: found
- Article: not found
Cancer treatment and survivorship statistics, 2016
- Record: found
- Abstract: found
- Article: not found