- Record: found
- Abstract: found
- Article: not found
Poly(amidoamine) Dendrimer–Methotrexate Conjugates: The Mechanism of Interaction with Folate Binding Protein
Read this article at
Abstract

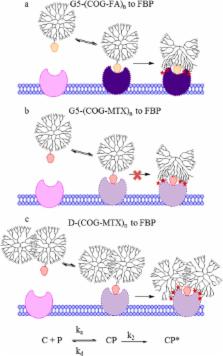
Generation 5 poly(amidoamine) (G5 PAMAM) methotrexate (MTX) conjugates employing two small molecular linkers, G5-(COG-MTX) n , G5-(MFCO-MTX) n were prepared along with the conjugates of the G5-G5 (D) dimer, D-(COG-MTX) n , D-(MFCO-MTX) n . The monomer G5-(COG-MTX) n conjugates exhibited only a weak, rapidly reversible binding to folate binding protein (FBP) consistent with monovalent MTX binding. The D-(COG-MTX) n conjugates exhibited a slow onset, tight-binding mechanism in which the MTX first binds to the FBP, inducing protein structural rearrangement, followed by polymer–protein van der Waals interactions leading to tight-binding. The extent of irreversible binding is dependent on total MTX concentration and no evidence of multivalent MTX binding was observed.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Dendrimers and dendritic polymers in drug delivery.
- Record: found
- Abstract: found
- Article: not found
Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications.
- Record: found
- Abstract: not found
- Article: not found
