- Record: found
- Abstract: found
- Article: found
Targeting intracellular B2 receptors using novel cell-penetrating antagonists to arrest growth and induce apoptosis in human triple-negative breast cancer
Read this article at
Abstract
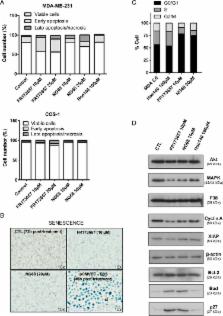
G protein-coupled receptors (GPCRs) are integral cell-surface proteins having a central role in tumor growth and metastasis. However, several GPCRs retain an atypical intracellular/nuclear location in various types of cancer. The pathological significance of this is currently unknown. Here we extend this observation by showing that the bradykinin B2R (BK-B2R) is nuclearly expressed in the human triple-negative breast cancer (TNBC) cell line MDA-MB-231 and in human clinical specimens of TNBC. We posited that these “nuclearized” receptors could be involved in oncogenic signaling linked to aberrant growth and survival maintenance of TNBC. We used cell-penetrating BK-B2R antagonists, including FR173657 and novel transducible, cell-permeable forms of the peptide B2R antagonist HOE 140 (NG68, NG134) to demonstrate their superior efficacy over impermeable ones (HOE 140), in blocking proliferation and promoting apoptosis of MDA-MB-231 cells. Some showed an even greater antineoplastic activity over conventional chemotherapeutic drugs in vitro. The cell-permeable B2R antagonists had less to no anticancer effects on B2R shRNA-knockdown or non-B2R expressing (COS-1) cells, indicating specificity in their action. Possible mechanisms of their anticancer effects may involve activation of p38kinase/p27 Kip1 pathways. Together, our data support the existence of a possible intracrine signaling pathway via internal/nuclear B2R, critical for the growth of TNBC cells, and identify new chemical entities that enable to target the corresponding intracellular GPCRs.
Related collections
Most cited references75

- Record: found
- Abstract: found
- Article: found
Choosing the right cell line for breast cancer research
- Record: found
- Abstract: found
- Article: not found
G-protein-coupled receptors and cancer.
- Record: found
- Abstract: found
- Article: not found