- Record: found
- Abstract: found
- Article: found
Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension

Read this article at
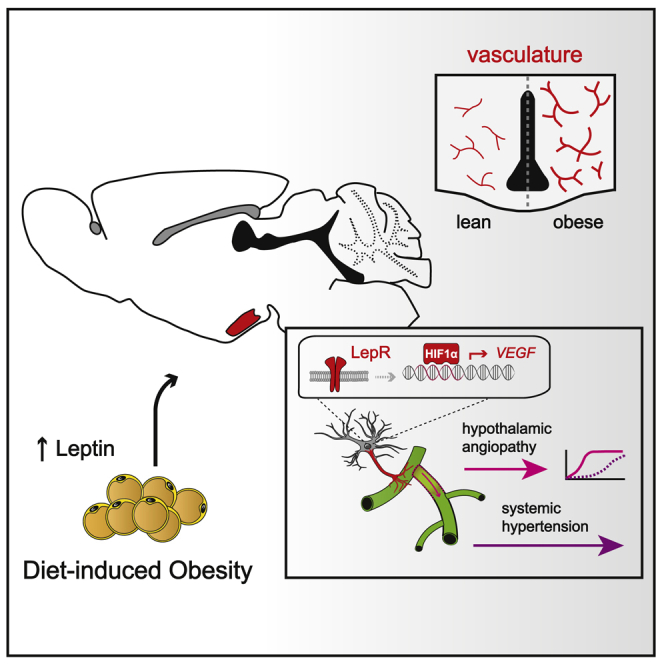
Summary
Pathologies of the micro- and macrovascular systems are a hallmark of the metabolic syndrome, which can lead to chronically elevated blood pressure. However, the underlying pathomechanisms involved still need to be clarified. Here, we report that an obesity-associated increase in serum leptin triggers the select expansion of the micro-angioarchitecture in pre-autonomic brain centers that regulate hemodynamic homeostasis. By using a series of cell- and region-specific loss- and gain-of-function models, we show that this pathophysiological process depends on hypothalamic astroglial hypoxia-inducible factor 1α-vascular endothelial growth factor (HIF1α-VEGF) signaling downstream of leptin signaling. Importantly, several distinct models of HIF1α-VEGF pathway disruption in astrocytes are protected not only from obesity-induced hypothalamic angiopathy but also from sympathetic hyperactivity or arterial hypertension. These results suggest that hyperleptinemia promotes obesity-induced hypertension via a HIF1α-VEGF signaling cascade in hypothalamic astrocytes while establishing a novel mechanistic link that connects hypothalamic micro-angioarchitecture with control over systemic blood pressure.
Graphical abstract
Highlights
-
•
The hypothalamic gliovascular interface is dynamically remodeled during obesity
-
•
Circulating leptin couples hypercaloric states with hypothalamic microangiopathy
-
•
Leptin-induced astroglial HIF1α-VEGF drives angiogenesis in the hypothalamus
-
•
VEGF in astrocytes promotes the development of arterial hypertension during obesity
Abstract
Here, Gruber et al. show that during diet-induced obesity in mice there is a profound remodeling of the gliovascular interface in the hypothalamus, resulting in arterial hypertension. This process is driven by elevated leptin levels and upregulation of a HIF1α-VEGF signaling axis in local astrocytes.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.
- Record: found
- Abstract: found
- Article: not found
A robust and high-throughput Cre reporting and characterization system for the whole mouse brain
- Record: found
- Abstract: not found
- Article: not found