- Record: found
- Abstract: found
- Article: found
LILRB4 regulates multiple myeloma development through STAT3-PFKFB1 pathway
Read this article at
Abstract
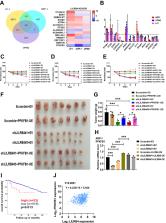
Although multiple myeloma (MM) responds well to immunotherapeutic treatment, certain portions of MM are still unresponsive or relapse after immunotherapy. Other immune molecules are needed for the immunotherapy of MM. Here, we revealed that leukocyte immunoglobulin-like receptor B4 (LILRB4) was highly expressed in multiple myeloma cell lines and patient samples and that the expression of LILRB4 was adversely correlated with the overall survival of MM patients. Knockdown of LILRB4 efficiently delayed the growth of MM cells both in vitro and in vivo. Mechanistically, IKZF1 transactivated LILRB4 expression to trigger the downstream of STAT3-PFKFB1 pathways to support MM cell proliferation. Blockade of LILRB4 signaling by blocking antibodies can effectively inhibit MM progression. Our data show that targeting LILRB4 is potentially an additional therapeutic strategy for the immunotherapeutic treatment of MM.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma.
- Record: found
- Abstract: found
- Article: not found
Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma
- Record: found
- Abstract: not found
- Article: not found