- Record: found
- Abstract: found
- Article: found
Efficacy of low-load resistance training combined with blood flow restriction vs. high-load resistance training on sarcopenia among community-dwelling older Chinese people: study protocol for a 3-arm randomized controlled trial
Read this article at
Abstract
Background
Sarcopenia is accompanied by a decline in muscle mass, muscle strength, and muscle function. Resistance training is the most potential training method for the prevention and treatment of sarcopenia. However, the conventional high-load resistance training (CRT) recommended by the American College of Sports Medicine is a challenge for older people with sarcopenia. As a novel training method, low-load resistance training combined with blood flow restriction (LRT-BFR) may elicit similar muscle mass and muscle strength gains as CRT but with less effort. The objectives of this study are to assess and compare the efficacy and safety of 12-week LRT-BFR and CRT on muscle strength, muscle performance, body composition, pulmonary function, blood biomarkers, CVD risk factors, and quality of life in community-dwelling older Chinese people with sarcopenia.
Method
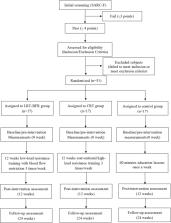
This is a 12-week, assessor-blinded, 3-arm randomized controlled trial with a non-exercise control group. Community-dwelling people over 65 years will be screened for sarcopenia according to the diagnostic criteria of the Asian Working Group for Sarcopenia (AWGS). Fifty-one subjects will be randomized into a LRT-BFR group ( n = 17), a CRT group ( n = 17), and a no-strength training control group ( n = 17). The primary outcome is lower limb muscle strength. The secondary outcomes are body composition, upper limb muscle strength, pulmonary function, blood biomarkers, CVD risk factors, and quality of life. Post-intervention follow-up will be performed for 12 weeks. These indicators will be assessed at baseline (0 week), after the 12-week intervention (12 weeks), and at follow-up (24 weeks). The adverse events will also be reported. Data will be analyzed for all participants in an intent-to-treat plan.
Discussion
This study is the first RCT that will systematically measure and compare the efficacy and safety of LRT-BFR and CRT in older people with sarcopenia on muscle strength, body composition, pulmonary function, blood biomarkers (inflammatory biomarkers, hormone, and growth factors), CVD risk factors, and quality of life. This study can provide an efficient and safe method to prevent the progression of sarcopenia in older people.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Sarcopenia: European consensus on definition and diagnosis
- Record: found
- Abstract: found
- Article: not found
Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment
- Record: found
- Abstract: not found
- Article: not found