- Record: found
- Abstract: found
- Article: found
Antenatal Glucocorticoid Treatment Induces Adaptations in Adult Midbrain Dopamine Neurons, which Underpin Sexually Dimorphic Behavioral Resilience
Read this article at
Abstract
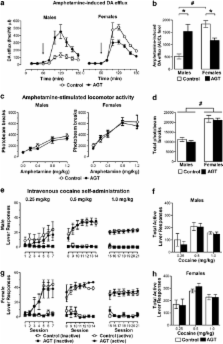
We demonstrated previously that antenatal glucocorticoid treatment (AGT, gestational days 16–19) altered the size and organization of the adult rat midbrain dopaminergic (DA) populations. Here we investigated the consequences of these AGT-induced cytoarchitectural disturbances on indices of DA function in adult rats. We show that in adulthood, enrichment of striatal DA fiber density paralleled AGT-induced increases in the numbers of midbrain DA neurons, which retained normal basal electrophysiological properties. This was co-incident with changes in (i) striatal D 2-type receptor levels (increased, both sexes); (ii) D 1-type receptor levels (males decreased; females increased); (iii) DA transporter levels (males increased; females decreased) in striatal regions; and (iv) amphetamine-induced mesolimbic DA release (males increased; females decreased). However, despite these profound, sexually dimorphic changes in markers of DA neurotransmission, in-utero glucocorticoid overexposure had a modest or no effect on a range of conditioned and unconditioned appetitive behaviors known to depend on mesolimbic DA activity. These findings provide empirical evidence for enduring AGT-induced adaptive mechanisms within the midbrain DA circuitry, which preserve some, but not all, functions, thereby casting further light on the vulnerability of these systems to environmental perturbations. Furthermore, they demonstrate these effects are achieved by different, often opponent, adaptive mechanisms in males and females, with translational implications for sex biases commonly found in midbrain DA-associated disorders.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines.
- Record: found
- Abstract: found
- Article: not found