- Record: found
- Abstract: found
- Article: found
Basophils beyond allergic and parasitic diseases
Read this article at
Abstract
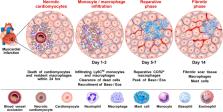
Basophils bind IgE via FcεRI-αβγ 2, which they uniquely share only with mast cells. In doing so, they can rapidly release mediators that are hallmark of allergic disease. This fundamental similarity, along with some morphological features shared by the two cell types, has long brought into question the biological significance that basophils mediate beyond that of mast cells. Unlike mast cells, which mature and reside in tissues, basophils are released into circulation from the bone marrow (constituting 1% of leukocytes), only to infiltrate tissues under specific inflammatory conditions. Evidence is emerging that basophils mediate non-redundant roles in allergic disease and, unsuspectingly, are implicated in a variety of other pathologies [e.g., myocardial infarction, autoimmunity, chronic obstructive pulmonary disease, fibrosis, cancer, etc.]. Recent findings strengthen the notion that these cells mediate protection from parasitic infections, whereas related studies implicate basophils promoting wound healing. Central to these functions is the substantial evidence that human and mouse basophils are increasingly implicated as important sources of IL-4 and IL-13. Nonetheless, much remains unclear regarding the role of basophils in pathology vs. homeostasis. In this review, we discuss the dichotomous (protective and/or harmful) roles of basophils in a wide spectrum of non-allergic disorders.
Related collections
Most cited references277
- Record: found
- Abstract: not found
- Article: not found
Severe Covid-19
- Record: found
- Abstract: found
- Article: not found
Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN.
- Record: found
- Abstract: found
- Article: not found