- Record: found
- Abstract: found
- Article: found
A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid
Read this article at
Abstract
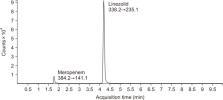
Antibiotic therapy requires appropriate dosage of drugs for effective treatment. Too low antibiotic concentrations may lead to treatment failure and the development of resistant pathogens, whereas overdosing may cause neurological side effects or hemolytic diseases. Meropenem and linezolid are used only in the treatment of serious infections or when other antibiotics are no longer effective as well as for treating central nervous system infections. It is difficult or sometimes even impossible to predict the relation between dosing of antibiotics and its cerebrospinal fluid (CSF) concentration; thus, a method of determining antibiotics not only in the blood but also in the CSF is needed. Analytical method validation is an integral part of good laboratory practice and ensures high accuracy of the results. We performed complete validation process according to the Food and Drug Administration and European Medicine Agency, covering the aspects precision, specificity, accuracy, recovery, limit of detection, limit of quantification, stability, carry-over, and matrix effects. Our liquid chromatography-tandem mass spectrometry method for the simultaneous measurement of meropenem and linezolid in different matrix meets all the acceptance criteria. The method was successfully applied to determine meropenem and linezolid concentrations in serum and CSF samples obtained from children treated with these antibiotics.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections.
- Record: found
- Abstract: not found
- Article: not found
LC-MS/MS method for simultaneous determination of linezolid, meropenem, piperacillin and teicoplanin in human plasma samples

- Record: found
- Abstract: found
- Article: found