- Record: found
- Abstract: found
- Article: found
Deoxyguanosine kinase deficiency: natural history and liver transplant outcome
Read this article at
Abstract
Autosomal recessive pathogenetic variants in the DGUOK gene cause deficiency of deoxyguanosine kinase activity and mitochondrial deoxynucleotides pool imbalance, consequently, leading to quantitative and/or qualitative impairment of mitochondrial DNA synthesis. Typically, patients present early-onset liver failure with or without neurological involvement and a clinical course rapidly progressing to death.
This is an international multicentre study aiming to provide a retrospective natural history of deoxyguanosine kinase deficient patients. A systematic literature review from January 2001 to June 2023 was conducted. Physicians of research centres or clinicians all around the world caring for previously reported patients were contacted to provide followup information or additional clinical, biochemical, histological/histochemical, and molecular genetics data for unreported cases with a confirmed molecular diagnosis of deoxyguanosine kinase deficiency.
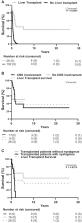
A cohort of 202 genetically confirmed patients, 36 unreported, and 166 from a systematic literature review, were analyzed. Patients had a neonatal onset (≤ 1 month) in 55.7% of cases, infantile (>1 month and ≤ 1 year) in 32.3%, pediatric (>1 year and ≤18 years) in 2.5% and adult (>18 years) in 9.5%. Kaplan-Meier analysis showed statistically different survival rates ( P < 0.0001) among the four age groups with the highest mortality for neonatal onset. Based on the clinical phenotype, we defined four different clinical subtypes: hepatocerebral (58.8%), isolated hepatopathy (21.9%), hepatomyoencephalopathy (9.6%), and isolated myopathy (9.6%). Muscle involvement was predominant in adult-onset cases whereas liver dysfunction causes morbidity and mortality in early-onset patients with a median survival of less than 1 year. No genotype–phenotype correlation was identified. Liver transplant significantly modified the survival rate in 26 treated patients when compared with untreated. Only six patients had additional mild neurological signs after liver transplant.
In conclusion, deoxyguanosine kinase deficiency is a disease spectrum with a prevalent liver and brain tissue specificity in neonatal and infantile-onset patients and muscle tissue specificity in adult-onset cases. Our study provides clinical, molecular genetics and biochemical data for early diagnosis, clinical trial planning and immediate intervention with liver transplant and/or nucleoside supplementation.
Abstract
Manzoni et al. demonstrated Deoxyguanosine kinase deficiency as a tissue-specific mitochondrial DNA maintenance defect manifesting with rapidly progressive liver disease in early-onset patients and mild myopathy in adults. Early recognition is essential for immediate intervention with a Liver transplant, showing evidence of efficacy in the analysed cohort, and/or nucleoside supplementation.
Graphical Abstract
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Use of whole-exome sequencing to determine the genetic basis of multiple mitochondrial respiratory chain complex deficiencies.
- Record: found
- Abstract: found
- Article: not found
Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease.
- Record: found
- Abstract: found
- Article: not found
