- Record: found
- Abstract: found
- Article: found
Cancer Immune Therapy for Philadelphia Chromosome-Negative Chronic Myeloproliferative Neoplasms
Read this article at
Abstract
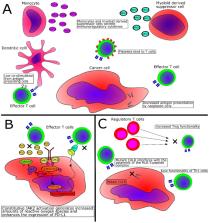
Philadelphia chromosome-negative chronic myeloproliferative neoplasms (MPN) are neoplastic diseases of the hematopoietic stem cells in the bone marrow. MPN are characterized by chronic inflammation and immune dysregulation. Of interest, the potent immunostimulatory cytokine interferon-α has been used to treat MPN for decades. A deeper understanding of the anti-cancer immune response and of the different immune regulatory mechanisms in patients with MPN has paved the way for an increased perception of the potential of cancer immunotherapy in MPN. Therapeutic vaccination targeting the driver mutations in MPN is one recently described potential new treatment modality. Furthermore, T cells can directly react against regulatory immune cells because they recognize proteins like arginase and programmed death ligand 1 (PD-L1). Therapeutic vaccination with arginase or PD-L1 therefore offers a novel way to directly affect immune inhibitory pathways, potentially altering tolerance to tumor antigens like mutant CALR and mutant JAK2. Other therapeutic options that could be used in concert with therapeutic cancer vaccines are immune checkpoint–blocking antibodies and interferon-α. For more advanced MPN, adoptive cellular therapy is a potential option that needs more preclinical investigation. In this review, we summarize current knowledge about the immune system in MPN and discuss the many opportunities for anti-cancer immunotherapy in patients with MPN.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Myeloid-derived suppressor cells: linking inflammation and cancer.
- Record: found
- Abstract: found
- Article: not found
Antitumour actions of interferons: implications for cancer therapy.
- Record: found
- Abstract: found
- Article: not found