- Record: found
- Abstract: found
- Article: found
Monitoring and Discussing Health-Related Quality of Life in Adolescents With Type 1 Diabetes Improve Psychosocial Well-Being : A randomized controlled trial
Read this article at
Abstract
OBJECTIVE—To test the effects of monitoring and discussing of health-related quality of life (HRQoL) in adolescents with type 1 diabetes in a multicenter randomized controlled trial.
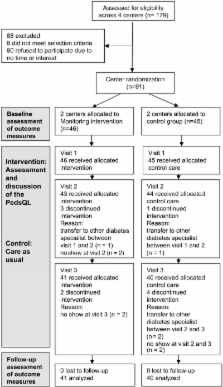
RESEARCH DESIGN AND METHODS—Four centers were randomly assigned to the HRQoL intervention (46 adolescents) or control (45 adolescents) group, with three regular visits scheduled within 12 months in both groups. In the HRQoL intervention group, HRQoL of adolescents was assessed using the Pediatric Quality of Life Inventory, and outcomes were discussed face-to-face during the consultation. The control group received care as usual. Mean differences between the groups at 12 months in physical and psychosocial well-being (Child Health Questionnaire [CHQ]-CF87/PF50, Diabetes-Specific Family Conflict Scale, and Center for Epidemiological Studies Scale for Depression), satisfaction with care (Patients’ Evaluation of the Quality of Diabetes Care), and A1C were determined, controlling for baseline scores.
RESULTS—Mean scores on the CHQ subscales of psychosocial health ( P < 0.001), behavior ( P < 0.001), mental health ( P < 0.001), and family activities ( P < 0.001) improved in the HRQoL intervention group, except for adolescents with the highest A1C values. Adolescents in the HRQoL intervention group reported higher self-esteem (CHQ) at follow-up ( P = 0.016), regardless of A1C, and were more satisfied with care ( P = 0.009) than control subjects. No significant differences between the two groups over time were observed in A1C levels.
CONCLUSIONS—Periodic monitoring and discussion of HRQoL in adolescents with diabetes is appreciated and has positive effects on their psychosocial well-being, except for those in poorest control.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module.
- Record: found
- Abstract: found
- Article: not found