- Record: found
- Abstract: found
- Article: found
Targeting PARP1 to Enhance Anticancer Checkpoint Immunotherapy Response: Rationale and Clinical Implications
Read this article at
Abstract
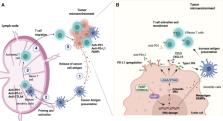
Reinvigorating the antitumor immune response using immune checkpoint inhibitors (ICIs) has revolutionized the treatment of several malignancies. However, extended use of ICIs has resulted in a cancer-specific response. In tumors considered to be less immunogenic, the response rates were low or null. To overcome resistance and improve the beneficial effects of ICIs, novel strategies focused on ICI-combined therapies have been tested. In particular, poly ADP-ribose polymerase inhibitors (PARPi) are a class of agents with potential for ICI combined therapy. PARPi impairs single-strand break DNA repair; this mechanism involves synthetic lethality in tumor cells with deficient homologous recombination. More recently, novel evidence indicated that PAPRi has the potential to modulate the antitumor immune response by activating antigen-presenting cells, infiltrating effector lymphocytes, and upregulating programmed death ligand-1 in tumors. This review covers the current advances in the immune effects of PARPi, explores the potential rationale for combined therapy with ICIs, and discusses ongoing clinical trials.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
Oncology meets immunology: the cancer-immunity cycle.
- Record: found
- Abstract: found
- Article: not found
Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer
- Record: found
- Abstract: found
- Article: not found