- Record: found
- Abstract: found
- Article: not found
COVID-19-associated fungal infections
Read this article at
Abstract
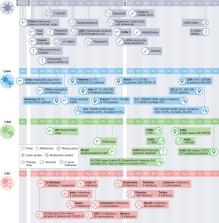
Coronavirus disease 2019 (COVID-19)-associated invasive fungal infections are an important complication in a substantial number of critically ill, hospitalized patients with COVID-19. Three groups of fungal pathogens cause co-infections in COVID-19: Aspergillus, Mucorales and Candida species, including Candida auris. Here we review the incidence of COVID-19-associated invasive fungal infections caused by these fungi in low-, middle- and high-income countries. By evaluating the epidemiology, clinical risk factors, predisposing features of the host environment and immunological mechanisms that underlie the pathogenesis of these co-infections, we set the scene for future research and development of clinical guidance.
Abstract
Hoenigl and colleagues review the epidemiology, immunology and clinical risk factors contributing to COVID-19-associated fungal infections.
Related collections
Most cited references161
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Record: found
- Abstract: found
- Article: not found
Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study
- Record: found
- Abstract: found
- Article: not found
