- Record: found
- Abstract: found
- Article: found
Sleep, Autonomic Nervous Function and Atherosclerosis
Read this article at
Abstract
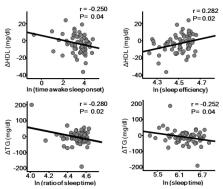
Behavioral and psychosocial factors related to development of cardiovascular disease have been gaining increased attention. Notably, sleep is considered to be one of the most important behavioral factors involved in progression of atherosclerosis and cardiovascular events, with autonomic nervous function a potential mechanism. Several studies have shown associations of sleep and autonomic dysfunction with major surrogate markers of atherosclerosis, such as carotid intima-media thickness and arterial stiffness. Endocrinological, immunological, oxidative, inflammatory, and metabolic responses, as well as endothelial dysfunction may mediate the effects of the autonomic nervous system. For this review, we examined recent findings related to sleep, autonomic nervous dysfunction, and atherosclerosis, with the aim of understanding the involved pathophysiological mechanisms.
Related collections
Most cited references72
- Record: found
- Abstract: found
- Article: not found
Impact of Reduced Heart Rate Variability on Risk for Cardiac Events: The Framingham Heart Study
- Record: found
- Abstract: found
- Article: not found
Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.
- Record: found
- Abstract: found
- Article: not found