- Record: found
- Abstract: found
- Article: found
The effect of letrozole versus artificial hormonal endometrial preparation on pregnancy outcome after frozen-thawed embryos transfer cycles: a randomized clinical trial
Read this article at
Abstract
Background
Considering that clinical trial studies are limited in polycystic ovary syndrome (PCOS) patients, and there is no consensus on an optimum endometrial preparation protocol for frozen embryo transfer (FET), the present study was designed as a randomized clinical trial to compare the reproductive outcomes following stimulated cycles with letrozole plus human menopausal gonadotropin (HMG) for endometrial preparation compared with routine AC-FET.
Methods
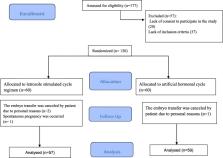
This randomized controlled trial was carried out on infertile PCOS patients who underwent IVF/ICSI and FET cycles in Arash Women’s Hospital affiliated to Tehran University of Medical Sciences between September 2018 and January 2020. PCOS diagnosis was based on the Rotterdam criteria. Eligible patients were randomly allocated into two groups: stimulated cycle with letrozole plus (HMG) (intervention group) and routine artificial hormonal endometrial preparation (control group).
Results
One hundred seventy-seven infertile patients were recruited for participation in the study. Of these, 57 women were excluded due to non-eligibility for entering the study, and a total of 120 patients were randomly assigned to two study groups. After follow up, the cycle outcomes of 57 patients in the intervention group and 59 patients in the control group were compared. The data analysis showed that the two groups did not have significant differences in fundamental and demographic characteristics. After the intervention, there were no significant differences in implantation rate, chemical, ectopic, and clinical pregnancy rates between groups. Moreover, the rates of miscarriage and ongoing pregnancy were similar between groups ( P > 0.05).
Conclusions
We found similar pregnancy outcomes with two endometrial preparation methods. Noting that each treatment centre should select the most beneficial and cost-effective method with the least adverse effects for patients, letrozole preparations for FET could be incorporated into possible options; however, establishing this approach as first-line treatment is premature in light of current evidence, and future randomized clinical trials with larger sample sizes are required for widespread application.
Trial registration
The study was also registered in the Iranian Registry of Clinical Trials on March 20th, 2020. ( IRCT20090526001952N12 at www.irct.ir, registered retrospectively).
Related collections
Most cited references22
- Record: found
- Abstract: not found
- Article: not found
A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality
- Record: found
- Abstract: found
- Article: not found
A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer.
- Record: found
- Abstract: found
- Article: found