- Record: found
- Abstract: found
- Article: found
Intestinal Epithelial Barrier Maturation by Enteric Glial Cells Is GDNF-Dependent
Read this article at
Abstract
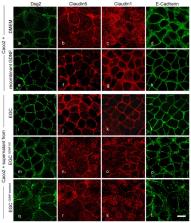
Enteric glial cells (EGCs) of the enteric nervous system are critically involved in the maintenance of intestinal epithelial barrier function (IEB). The underlying mechanisms remain undefined. Glial cell line-derived neurotrophic factor (GDNF) contributes to IEB maturation and may therefore be the predominant mediator of this process by EGCs. Using GFAP cre x Ai14 floxed mice to isolate EGCs by Fluorescence-activated cell sorting (FACS), we confirmed that they synthesize GDNF in vivo as well as in primary cultures demonstrating that EGCs are a rich source of GDNF in vivo and in vitro. Co-culture of EGCs with Caco2 cells resulted in IEB maturation which was abrogated when GDNF was either depleted from EGC supernatants, or knocked down in EGCs or when the GDNF receptor RET was blocked. Further, TNFα-induced loss of IEB function in Caco2 cells and in organoids was attenuated by EGC supernatants or by recombinant GDNF. These barrier-protective effects were blunted when using supernatants from GDNF-deficient EGCs or by RET receptor blockade. Together, our data show that EGCs produce GDNF to maintain IEB function in vitro through the RET receptor.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: found
Intestinal permeability – a new target for disease prevention and therapy

- Record: found
- Abstract: found
- Article: found
CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering

- Record: found
- Abstract: found
- Article: found