- Record: found
- Abstract: found
- Article: found
The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis
Read this article at
Abstract
Cilia intraflagellar transport and ciliogenesis are regulated by two small GTPases that maintain binding between IFT subcomplexes.
Abstract
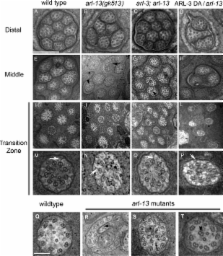
Intraflagellar transport (IFT) machinery mediates the bidirectional movement of cargos that are required for the assembly and maintenance of cilia. However, little is known about how IFT is regulated in vivo. In this study, we show that the small guanosine triphosphatase (GTPase) adenosine diphosphate ribosylation factor–like protein 13 (ARL-13) encoded by the Caenorhabditis elegans homologue of the human Joubert syndrome causal gene ARL13B, localizes exclusively to the doublet segment of the cilium. arl-13 mutants have shortened cilia with various ultrastructural deformities and a disrupted association between IFT subcomplexes A and B. Intriguingly, depletion of ARL-3, another ciliary small GTPase, partially suppresses ciliogenesis defects in arl-13 mutants by indirectly restoring binding between IFT subcomplexes A and B. Rescue of arl-13 mutants by ARL-3 depletion is mediated by an HDAC6 deacetylase-dependent pathway. Thus, we propose that two conserved small GTPases, ARL-13 and ARL-3, coordinate to regulate IFT and that perturbing this balance results in cilia deformation.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
The ciliopathies: an emerging class of human genetic disorders.
- Record: found
- Abstract: found
- Article: not found
Mutant sensory cilia in the nematode Caenorhabditis elegans.
- Record: found
- Abstract: found
- Article: not found