- Record: found
- Abstract: found
- Article: found
Causes of impaired oral vaccine efficacy in developing countries
Read this article at
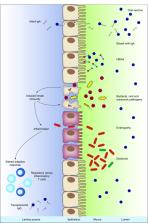
Abstract
Oral vaccines are less immunogenic when given to infants in low-income compared with high-income countries, limiting their potential public health impact. Here, we review factors that might contribute to this phenomenon, including transplacental antibodies, breastfeeding, histo blood group antigens, enteric pathogens, malnutrition, microbiota dysbiosis and environmental enteropathy. We highlight several clear risk factors for vaccine failure, such as the inhibitory effect of enteroviruses on oral poliovirus vaccine. We also highlight the ambiguous and at times contradictory nature of the available evidence, which undoubtedly reflects the complex and interconnected nature of the factors involved. Mechanisms responsible for diminished immunogenicity may be specific to each oral vaccine. Interventions aiming to improve vaccine performance may need to reflect the diversity of these mechanisms.
Related collections
Most cited references172
- Record: found
- Abstract: found
- Article: not found
Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis.
- Record: found
- Abstract: found
- Article: not found
Effect of human rotavirus vaccine on severe diarrhea in African infants.
- Record: found
- Abstract: found
- Article: not found