- Record: found
- Abstract: found
- Article: found
A Phase I Study to Evaluate the Pharmacokinetics and Safety of Cabotegravir in Adults With Severe Renal Impairment and Healthy Matched Control Participants
Read this article at
Abstract
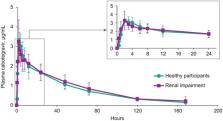
This study investigates the impact of severe renal impairment on the pharmacokinetics of cabotegravir, an investigational HIV‐1 integrase inhibitor. This was a phase I, open‐label, parallel‐group, multicenter study conducted in 8 participants with severe renal impairment (creatinine clearance <30 mL/min; no renal replacement therapy) and 8 healthy participants (creatinine clearance >90 mL/min; 2 women/group; 6 men/group) matched for sex, age (±10 years), and body mass index (±25%). Participants received a single 30‐mg oral cabotegravir tablet to determine total and unbound plasma cabotegravir concentrations. Arithmetic and geometric least squares means were calculated, and cabotegravir noncompartmental pharmacokinetic parameters were compared using geometric least squares mean ratios with 90% confidence intervals. Safety was assessed throughout the study. Geometric least squares mean ratios (90% confidence intervals) were 0.97 (0.84–1.14) for area under the plasma concentration‐time curve extrapolated to infinity, 1.01 (0.87–1.17) for maximum observed plasma concentration, 1.31 (0.84–2.03) for unbound cabotegravir 2 hours after dosing, and 1.51 (1.19–1.92) for unbound cabotegravir 24 hours after dosing. All adverse events were grade 1, except grade 3 lipase elevation in a participant with renal impairment. Severe renal impairment did not impact plasma cabotegravir exposure, and cabotegravir may be administered without dose adjustment in renal impairment among patients not receiving renal replacement.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial
- Record: found
- Abstract: not found
- Article: not found
Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America.

- Record: found
- Abstract: found
- Article: found
Long-acting injectable antiretrovirals for HIV treatment and prevention
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
