- Record: found
- Abstract: found
- Article: found
Correction: Epitope Mapping of Antibodies Suggests the Novel Membrane Topology of B-Cell Receptor Associated Protein 31 on the Cell Surface of Embryonic Stem Cells: The Novel Membrane Topology of BAP31
correction
6 January 2017
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
In Fig 5A, the N-terminal residue of the underlined epitope is incorrectly listed
as Glutamate (E) instead of Glutamine (Q). Please see the corrected Fig 5 here.
10.1371/journal.pone.0170145.g001
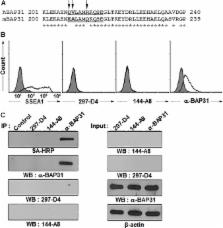
Fig 5
BAP31 is also expressed on the surface of mESCs.
(A) The epitope sequences of 297-D4 and 144-A8 are different between hESCs and mESCs.
The partial C-terminal amino acid sequences were aligned between human and mouse BAP31.
The epitope sequences are underlined, and the different amino acids are indicated
by arrows. (B) Flow cytometry analysis of mESCs with anti-SSEA-1, 297-D4, 144-A8,
and α-BAP31 antibodies. (C) Immunoprecipitation of mESCs with 297-D4, 144-A8, and
α-BAP31 antibodies. Cell surface proteins of mESCs were biotinylated, immuoprecipitated,
and analyzed by SA-HRP or western blot with α-BAP31 (left panels). The input samples
were also analyzed by western blots (right panels). β-actin was used as a loading
control.
