- Record: found
- Abstract: found
- Article: found
EphB/ephrinB Signaling in Cell Adhesion and Migration
Read this article at
Abstract
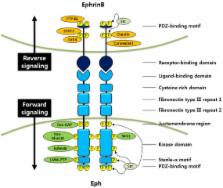
Eph receptors and their ligands, ephrins, represent the largest group of the receptor tyrosine kinase (RTK) family, and they mediate numerous developmental processes in a variety of organisms. Ephrins are membrane-bound proteins that are mainly divided into two classes: A class ephrins, which are linked to the membrane by a glycosylphosphatidylinositol (GPI) linkage, and B class ephrins, which are transmembrane ligands. Based on their domain structures and affinities for ligand binding, the Eph receptors are also divided into two groups. Trans-dimerization of Eph receptors with their membrane-tethered ligands regulates cell-cell interactions and initiates bidirectional signaling pathways. These pathways are intimately involved in regulating cytoskeleton dynamics, cell migration, and alterations in cellular dynamics and shapes. The EphBs and ephrinBs are specifically localized and modified to promote higher-order clustering and initiate of bidirectional signaling. In this review, we present an in-depth overview of the structure, mechanisms, cell signaling, and functions of EphB/ephrinB in cell adhesion and migration.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells.
- Record: found
- Abstract: found
- Article: not found
Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4.
- Record: found
- Abstract: found
- Article: not found