- Record: found
- Abstract: found
- Article: found
Humanized Mouse Models for the Study of Periodontitis: An Opportunity to Elucidate Unresolved Aspects of Its Immunopathogenesis and Analyze New Immunotherapeutic Strategies
Read this article at
Abstract
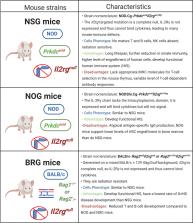
Periodontitis is an oral inflammatory disease in which the polymicrobial synergy and dysbiosis of the subgingival microbiota trigger a deregulated host immune response, that leads to the breakdown of tooth-supporting tissues and finally tooth loss. Periodontitis is characterized by the increased pathogenic activity of T helper type 17 (Th17) lymphocytes and defective immunoregulation mediated by phenotypically unstable T regulatory (Treg), lymphocytes, incapable of resolving the bone-resorbing inflammatory milieu. In this context, the complexity of the immune response orchestrated against the microbial challenge during periodontitis has made the study of its pathogenesis and therapy difficult and limited. Indeed, the ethical limitations that accompany human studies can lead to an insufficient etiopathogenic understanding of the disease and consequently, biased treatment decision-making. Alternatively, animal models allow us to manage these difficulties and give us the opportunity to partially emulate the etiopathogenesis of periodontitis by inoculating periodontopathogenic bacteria or by placing bacteria-accumulating ligatures around the teeth; however, these models still have limited translational application in humans. Accordingly, humanized animal models are able to emulate human-like complex networks of immune responses by engrafting human cells or tissues into specific strains of immunodeficient mice. Their characteristics enable a viable time window for the study of the establishment of a specific human immune response pattern in an in vivo setting and could be exploited for a wider study of the etiopathogenesis and/or treatment of periodontitis. For instance, the antigen-specific response of human dendritic cells against the periodontopathogen Porphyromonas gingivalis favoring the Th17/Treg response has already been tested in humanized mice models. Hypothetically, the proper emulation of periodontal dysbiosis in a humanized animal could give insights into the subtle molecular characteristics of a human-like local and systemic immune response during periodontitis and support the design of novel immunotherapeutic strategies. Therefore, the aims of this review are: To elucidate how the microbiota-elicited immunopathogenesis of periodontitis can be potentially emulated in humanized mouse models, to highlight their advantages and limitations in comparison with the already available experimental periodontitis non-humanized animal models, and to discuss the potential translational application of using these models for periodontitis immunotherapeutics.
Related collections
Most cited references137
- Record: found
- Abstract: found
- Article: not found
Periodontitis: from microbial immune subversion to systemic inflammation.
- Record: found
- Abstract: found
- Article: not found
Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry.
- Record: found
- Abstract: found
- Article: not found