- Record: found
- Abstract: found
- Article: found
Penetration Enhancers in Ocular Drug Delivery
Read this article at
Abstract
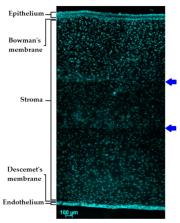
There are more than 100 recognized disorders of the eye. This makes the development of advanced ocular formulations an important topic in pharmaceutical science. One of the ways to improve drug delivery to the eye is the use of penetration enhancers. These are defined as compounds capable of enhancing drug permeability across ocular membranes. This review paper provides an overview of anatomical and physiological features of the eye and discusses some common ophthalmological conditions and permeability of ocular membranes. The review also presents the analysis of literature on the use of penetration-enhancing compounds (cyclodextrins, chelating agents, crown ethers, bile acids and bile salts, cell-penetrating peptides, and other amphiphilic compounds) in ocular drug delivery, describing their properties and modes of action.
Related collections
Most cited references113
- Record: found
- Abstract: found
- Article: not found
Bile acids: regulation of synthesis.
- Record: found
- Abstract: not found
- Article: not found
Cyclic polyethers and their complexes with metal salts
- Record: found
- Abstract: found
- Article: not found