- Record: found
- Abstract: found
- Article: found
Design of dual peptide-conjugated hydrogels for proliferation and differentiation of human pluripotent stem cells

Read this article at
Abstract
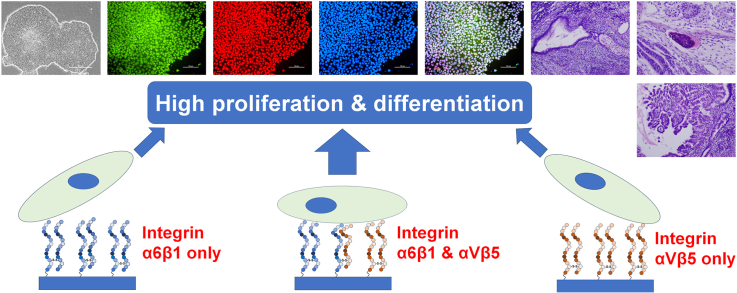
Completely synthetic cell cultivation materials for human pluripotent stem cells (hPSCs) are important for the future clinical use of hPSC-derived cells. Currently, cell culture materials conjugated with extracellular matrix (ECM)-derived peptides are being prepared using only one specific integrin-targeting peptide. We designed dual peptide-conjugated hydrogels, for which each peptide was selected from different ECM sites: the laminin β4 chain and fibronectin or vitronectin, which can target α6β1 and α2β1 or αVβ5. hPSCs cultured on dual peptide-conjugated hydrogels, especially on hydrogels conjugated with peptides obtained from the laminin β4 chain and vitronectin with a low peptide concentration of 200 μg/mL, showed high proliferation ability over the long term and differentiated into cells originating from 3 germ layers in vivo as well as a specific lineage of cardiac cells. The design of grafting peptides was also important, for which a joint segment and positive amino acids were added into the designed peptide. Because of the designed peptides on the hydrogels, only 200 μg/mL peptide solution was sufficient for grafting on the hydrogels, and the hydrogels supported hPSC cultures long-term; in contrast, in previous studies, greater than 1000 μg/mL peptide solution was needed for the grafting of peptides on cell culture materials.
Graphical abstract
Highlights
Human pluripotent stem cells can proliferate on dual peptide-grafted hydrogels.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Chemically Defined and Small Molecule-Based Generation of Human Cardiomyocytes
- Record: found
- Abstract: found
- Article: not found
Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells.
- Record: found
- Abstract: found
- Article: not found