- Record: found
- Abstract: found
- Article: found
Modeling polymorphic ventricular tachycardia at rest using patient-specific induced pluripotent stem cell-derived cardiomyocytes
Read this article at
Abstract
Background
While mutations in the cardiac type 2 ryanodine receptor (RyR2) have been linked to exercise-induced or catecholaminergic polymorphic ventricular tachycardia (CPVT), its association with polymorphic ventricular tachycardia (PMVT) occurring at rest is unclear. We aimed at constructing a patient-specific human-induced pluripotent stem cell (hiPSC) model of PMVT occurring at rest linked to a single point mutation in RyR2.
Methods
Blood samples were obtained from a patient with PMVT at rest due to a heterozygous RyR2-H29D mutation. Patient-specific hiPSCs were generated from the blood samples, and the hiPSC-derived cardiomyocytes (CMs) were generated via directed differentiation. Using CRIPSR/Cas9 technology, isogenic controls were generated by correcting the RyR2-H29D mutation. Using patch-clamp, fluorescent confocal microscopy and video-image-based analysis, the molecular and functional properties of RyR2-H29D hiPSC—CMs and control hiPSC—CMs were compared.
Findings
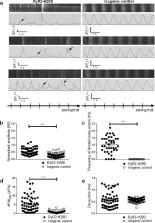
RyR2-H29D hiPSC—CMs exhibit intracellular sarcoplasmic reticulum (SR) Ca 2+ leak through RyR2 under physiological pacing. RyR2-H29D enhances the contribution of inositol 1,4,5-trisphosphate receptors to excitation-contraction coupling (ECC) that exacerbates abnormal Ca 2+ release in RyR2-H29D hiPSC—CMs. RyR2-H29D hiPSC—CMs exhibit shorter action potentials, delayed afterdepolarizations, arrhythmias and aberrant contractile properties compared to isogenic controls. The RyR2-H29D mutation causes post-translational remodeling that is fully reversed with isogenic controls.
Interpretation
To conclude, in a model based on a RyR2 point mutation that is associated with short-coupled PMVT at rest, RyR2-H29D hiPSC—CMs exhibited aberrant intracellular Ca 2+ homeostasis, shortened action potentials, arrhythmias and abnormal contractile properties.
Funding
French Muscular Dystrophy Association (AFM; project 16,073, MNM2 2012 and 20,225), “Fondation de la Recherche Médicale” (FRM; SPF20130526710), “Institut National pour la Santé et la Recherche Médicale” (INSERM), National Institutes of Health (ARM; R01 HL145473) and New York State Department of Health (NYSTEM C029156).
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Structure of a mammalian ryanodine receptor
- Record: found
- Abstract: found
- Article: not found
Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans.
- Record: found
- Abstract: found
- Article: not found