- Record: found
- Abstract: found
- Article: found
Casein kinase 1α: biological mechanisms and theranostic potential
Read this article at
Abstract
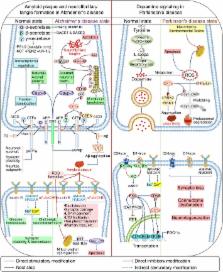
Casein kinase 1α (CK1α) is a multifunctional protein belonging to the CK1 protein family that is conserved in eukaryotes from yeast to humans. It regulates signaling pathways related to membrane trafficking, cell cycle progression, chromosome segregation, apoptosis, autophagy, cell metabolism, and differentiation in development, circadian rhythm, and the immune response as well as neurodegeneration and cancer. Given its involvement in diverse cellular, physiological, and pathological processes, CK1α is a promising therapeutic target. In this review, we summarize what is known of the biological functions of CK1α, and provide an overview of existing challenges and potential opportunities for advancing theranostics.
Related collections
Most cited references202
- Record: found
- Abstract: found
- Article: not found
Cell cycle proteins as promising targets in cancer therapy
- Record: found
- Abstract: found
- Article: not found
DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival.
- Record: found
- Abstract: found
- Article: not found