- Record: found
- Abstract: found
- Article: not found
The Induction of Tolerance by Dendritic Cells That Have Captured Apoptotic Cells
article-commentary
7 February 2000
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
What makes a protein immunogenic, particularly for strong T cell–mediated immunity?
To a first approximation, this determination seems to be made by dendritic cells (DCs).
Immature DCs, as in skin 1
2
3
4, lung 5, blood 6
7, and spleen 7
8, take up proteins, immune complexes, microbes, and dying cells. However, in order
to use these antigens to stimulate a T cell response, the DCs must undergo a characteristic
process of terminal differentiation called “maturation.” The known stimuli for DC
maturation are numerous and include inflammatory cytokines, CD40 ligand (CD40L), viral
and microbial constituents such as double-stranded RNA and LPS, and certain CpG oligonucleotides.
DC Maturation as a Control Point in the Initiation of Immunity
Maturation changes DCs in many ways that help explain their potent immunogenicity.
Examples include de novo expression of T cell costimulatory molecules like CD86 9
10; the capacity to produce IL-12 11
12 and resist immunosuppression by IL-10 13; the development of a new repertoire of
chemokine receptors, especially CCR7 14
15
16
17, that guide entry into lymphatics and migration to the T cell areas 18; the production
of DC survival and stimulatory molecules like CD40 and TNF-related activation-induced
cytokine receptor (TRANCE-R) 19
20; and a redistribution of MHC class II molecules from lysosomes to the cell surface
21
22. Recently, it has been found (Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama,
C. Reis e Sousa, R.N. Germain, J. Mellman, and R.M. Steinman, manuscript submitted
for publication) that immature DCs endocytose proteins into MHC class II–rich intracellular
compartments, but the cells must also mature to form MHC–peptide complexes there and
to export the complexes to the cell surface together with B7 costimulators. DC maturation
is understandably a key control point in converting an antigen into an immunogen.
The role for DCs in determining immunogenicity seems well established, but many are
now pursuing their role in other contexts: immune deviation, i.e., skewing T cells
to the Th2 phenotype; immune regulation, i.e., inducing Tr1 cells that make IL-10;
and bona fide tolerance, i.e., deletion and anergy. We will comment on the idea that
the uptake of dying cells by immature DCs is critical for the maintenance of peripheral
tolerance, including the findings in two papers in this issue. Huang et al. demonstrate
that DCs in afferent lymph carry apoptotic bodies derived from the intestinal epithelium
23. They present evidence, using an isoform of intestinal nonspecific esterase, that
DCs continually deliver samples of this tissue to the lymph node. Sauter et al. find
that DCs phagocytose apoptotic and necrotic cell lines, but only the latter cause
DCs to mature into strong stimulators of T cell immunity 24. Both papers suggest that
the uptake of apoptotic cells allows DCs to induce peripheral tolerance to self. We
will first outline why it makes sense for DCs, such potent agents of immunity, to
also ensure tolerance to cell-associated self-antigens that unavoidably are present
at sites of foreign antigen deposition.
The Value of Peripheral Tolerance Induction by DCs
Central or thymic tolerance is likely to be mediated by thymic DCs 25
26, but these DCs may not be able to delete T cells that react with many self-antigens
in peripheral tissues. Many proteins may not have access to the thymus during development,
especially antigens that are expressed after the thymus has generated a T cell repertoire,
e.g., breast constituents that are first expressed at puberty 27. Therefore, autoreactive
T cells that are not deleted in the thymus need to be silenced in the periphery to
prevent immune responses to self-tissues.
We would argue that it is essential for DCs to play a role in the induction of peripheral
tolerance. The reasoning is as follows. Maturing DCs have the capacity to process
and present peptides from dying cells to CD4 and CD8 T lymphocytes 28
29
30
31. In fact, DCs may be the principal cells that present antigens from dying cells
(“cross presentation”; see below). In this light, consider what might occur during
influenza infection of the airway (Fig. 1). During infection, there is extensive death
of virus-infected, airway epithelial cells or “self.” How do DCs focus immunity on
the virus, when they also should be presenting self-antigens from the infected, apoptotic,
airway epithelial cells (29
32; Fig. 1)? Because cell death is a feature of many infections, the danger of what
Ehrlich rightly termed “horror autotoxicus” is hardly limited to influenza.
Thus, peripheral tolerance to those peptides that can be processed from dying cells
seems critical for preventing autoreactivity, but when and how does this occur? It
would be valuable for DCs to induce peripheral tolerance to dying noninfected tissues,
since this would inactivate the key self-reactive T cells before the DCs are called
upon to initiate immunity to microbial antigens. In effect, peripheral tolerance should
share with central thymic tolerance the capacity to self-tolerize before foreign antigen
exposure and to use the same APC that will later be called upon to initiate immunity.
Peripheral Tolerance to Tissue Antigens Via Bone Marrow–derived Cells in Draining
Lymph Nodes
Precise tools have been developed to study peripheral tolerance. Neo self-antigens
are expressed as transgenes in peripheral tissues, and then the animal is injected
with the corresponding antigen-reactive, TCR transgenic T cells. Adler et al. 33
expressed the influenza hemagglutinin (HA) in many tissues. When HA-reactive CD4+
T cells were injected, the T cells were anergized, and when bone marrow chimeras were
examined, the marrow-derived cells had to express the MHC that was recognized by the
anergized, HA-reactive, TCR transgenic T cells 33. Anergy did not develop if only
nonhematopoietic tissue cells expressed the appropriate MHC. In similarly elegant
studies, Kurts et al. 34
35 expressed OVA sequences in insulin-producing β cells of pancreatic islets (Fig.
2). The OVA antigen in tissue cells was again presented to T cells by marrow-derived
cells 34, and the TCR transgenic CD8+ T cells seemed to be tolerized by deletion after
a series of cell divisions 35. Kurts et al. showed that the tolerizing, marrow-derived
cells were confined to the draining lymph nodes (Fig. 2), i.e., the nodes that received
afferent lymphatics from the tissue expressing the OVA antigen (the pancreas or kidney
in their studies). Analogous results have been reported by others 36
37
38.
Somehow then, self-antigens in peripheral tissues are transferred to marrow-derived
cells in a lymph node, and this can tolerize adult T cells. Although the marrow-derived
cells have yet to be pinpointed, DCs are a likely candidate since they comprise a
link between the peripheral tissues and the lymph node, the latter being the site
where the tolerizing self-signals appear to be presented.
The Capture of Tissue Cells by DCs
Phagocytic inclusions have been described previously in DCs that traffic from tissues
to lymph nodes in afferent lymph 39
40
41. Huang et al. now show that these inclusions are apoptotic bodies 23. Furthermore,
their new data indicate that the apoptotic bodies derive from intestinal epithelium,
presumably picked up from epithelial cells undergoing normal cell turnover.
The only comparable description of phagocytic inclusions in DCs in situ is a report
involving NK cell–mediated clearance of allogeneic leukocytes 42. DCs may also take
up apoptotic bodies during negative selection in the thymic medulla 43, but one cannot
visualize this, presumably because the digestion of apoptotic cells is so rapid 28.
Likewise, it is difficult to identify macrophages with phagocytosed dying cells in
situ. For example, many developing thymocytes undergo apoptosis if they fail to be
positively selected. The thymocytes are likely to be scavenged by macrophages in the
cortex, but this is only evident histologically if massive thymocyte death is induced
with steroids or irradiation 43.
Therefore, the sighting by Huang et al. 23 of apoptotic epithelial cells in mesenteric
lymph DCs suggests a major flux of tissue antigens via DCs that are heading to lymph
nodes. Immature DCs or their precursors may always be trafficking through tissues
44
45, picking up apoptotic material from cells undergoing the turnover that is characteristic
of most tissues. If the events described by Huang et al. 23 silence reactivity to
the intestinal peptides that are processed from dying cells, then DCs maturing during
a subsequent intestinal infection would only stimulate a response to foreign antigen,
thus alleviating the problem posed in Fig. 1.
Processing of Apoptotic Cells onto MHC Class I and II Products
Formation of MHC class I–peptide complexes from antigens in endocytosed dying cells
29
30 illustrates phenomena termed the “exogenous pathway” and the “cross-presentation”
of antigens. In the exogenous pathway, MHC class I molecules present peptides derived
from endocytosed proteins, rather than newly synthesized (“endogenous”) proteins in
the cytoplasm. One example of the exogenous pathway is cross-presentation, since exogenous
peptides from cells of one MHC, or even xenogeneic MHC, are presented by DCs of a
different MHC.
DCs efficiently carry out the exogenous pathway for MHC class I. This applies to peptides
derived from immune complexes 46, bacteria 47, and apoptotic cells dying because
of viral 29
30 or bacterial 31 infection. Rodriguez et al. have shown that molecules with molecular
masses of 3–20 kD somehow can escape the endocytic system of DCs into the cytoplasm
48. They postulate that the endocytic vacuoles of DCs have a transporter or pore whereby
substrates gain access to TAP molecules in the endoplasmic reticulum, followed by
presentation on MHC class I. The recent results from the Bhardwaj and Amigorena laboratories
also provide evidence that the exogenous pathway is expressed much more efficiently
in DCs than in macrophages and B cells 30
46
48.
Effects of Apoptotic Cells on DC Maturation
The paper by Sauter et al. in this issue introduces the critical events of DC maturation
to this topic. The uptake of apoptotic cells in the steady state must not mature the
DCs if these cells are to induce tolerance rather than immunity, and indeed this is
what Sauter et al. 24 and Gallucci et al. 49 now report. Immature DCs selectively
carry out phagocytosis of apoptotic cells 28
30, as is also the case for the uptake of microbes, latex, and immune complexes 4
46
50. For one thing, relevant phagocytic receptors are better expressed on immature
DCs, e.g., αVβ5 integrin for apoptotic bodies and FcγR for immune complexes 30
46.
If DCs only take up apoptotic cells when immature 28
30, if apoptotic cells do not mature the DCs 24, and if immature DCs are poor stimulators
of immunity 1, then what are the immunological consequences to the carriage of large
numbers of dying somatic cells by DCs in lymph 23? Is uptake immunologically “null,”
like the clearance of apoptotic bodies by macrophages, or might peripheral tolerance
ensue?
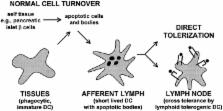
Hypothesis: Immature DCs Phagocytose Tissue Cells Undergoing Normal Cell Turnover
by Apoptosis; This Leads to Tolerance or Regulation of Self-reactive, Adult T Cells
in the Draining Lymph Node
In the steady state, i.e., in the absence of inflammation, infection, and necrosis,
DCs are always found in afferent lymph, where they are also called “veiled cells.”
Veiled cells might derive from precursors in the blood 6
51 including monocytes 44
45. The idea is that circulating immature DCs and monocytes can traffic through tissues,
picking up cells that die by apoptosis 28
30, and then enter the afferent lymph (Fig. 3). In the steady state, these DCs will
not receive maturation stimuli and therefore will be unable to stimulate immunity
to the self-antigens they have captured.
How might immature DCs induce tolerance to self-antigens in phagocytosed apoptotic
cells? One view is that migratory immature DCs tolerize T cells directly because of
a lack of costimulators (Fig. 3). There are potential difficulties with this idea.
For example, we have just found that immature DCs do not process endocytosed antigens
well to form MHC–peptide complexes (Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S.
Shimoyama, C. Reis e Sousa, R.N. Germain, J. Mellman, and R.M. Steinman, manuscript
submitted for publication). Therefore, self-reactive T cells would not be able to
recognize their ligand on immature DCs. Also, the immature DCs may lack the CD40 and
TRANCE-R that sustain DC viability for the 3–4 d needed before T cell tolerance becomes
evident 35
37. In contrast, as summarized above, mature DCs express high levels of MHC–peptide,
as well as the CD40 and TRANCE-R that sustain DC viability during the interaction
with activated T cells 52. Possibly the migratory immature DCs overcome some of these
potential shortcomings and develop their tolerizing function upon encountering T cells
or other stimuli after reaching the node.
A second mechanism is that there will be subsets of DCs, as first proposed by Suss
and Shortman 53, that are somehow specialized to regulate immunity or to induce tolerance.
Direct evidence for this DC subset remains elusive. However, the idea is that immature
DCs capture apoptotic bodies peripherally and transfer tissue-derived peptides to
tolerogenic DCs upon reaching the lymph node. We are intrigued by this possibility
because of the information that migratory DCs in lymph are short lived and appear
to be processed by longer-lived, resident DCs in lymph node 28. When migratory DCs
are injected into mice, the cells leave the injection site 54, presumably via the
afferent lymph, but <1% of the injected cells can be recovered 2 d later in a lymph
node according to new data from Josien et al. 55. The dying, injected DCs are not
totally destroyed by some “big Mac,” but instead can be processed and presented by
DCs in the lymph node 28. The lymph node DCs can express high levels of MHC–peptide
and interestingly, relatively low levels of surface CD86 costimulator 56. If these
lymph node DCs efficiently form MHC–peptide complexes from incoming DCs and their
contents, the former subset may be the best candidate to present self-antigens from
apoptotic cells in a tolerogenic way. We postulate that there are resident, lymph
node DCs which in the steady state induce tolerance to antigens in apoptotic bodies
carried by migratory lymph DCs (Fig. 3).
Tolerogenic DCs may constitute a separate differentiation pathway, as suggested by
Suss and Shortman 53. Perhaps these DCs derive from the distinct plasmacytoid precursor
termed DC2 by Liu and colleagues 57
58. The tolerizing function of DCs may be quite sophisticated. For example, tolerance
could ensue by deletion or anergy of the self-reactive T cell, as suggested by the
work of Adler et al. 33 and Kurts et al. 34
35, discussed above. Alternatively, DCs might expand regulatory T cells. The latter
may be needed for self-antigens at body surfaces like the intestine and airway, which
are unlikely to be devoid of DC maturation stimuli.
Conclusion
DCs are specialized to control immunity, to trigger immune responses, and also, it
appears, to maintain tolerance. These two spheres become intimately linked when one
appreciates that cell death often accompanies infection and that DCs can present self-antigens
from dying cells. The maturation of peripheral DCs, which is often triggered by infectious
agents, should allow at least some phagocytosed self-antigens to become immunogenic.
We develop the hypothesis that immature DCs in the steady state are inducing tolerance
to self-antigens within phagocytosed apoptotic bodies, derived from the normal turnover
of tissues. This occurs well before the entry of a foreign antigen, so when infection
and DC maturation take place, the immune system can focus on the foreign peptides
that the DCs have processed.
Sauter et al. 24 report that the uptake of apoptotic cells does not directly mature
DCs. Also Huang et al. 23 find that intestinal lymph DCs normally carry phagocytosed,
apoptotic, intestinal epithelial cells towards the lymph node, presumably without
inducing intestinal autoimmunity. It is known that marrow-derived cells within lymph
nodes can tolerize T cells to peptides synthesized in other tissues. Thus, DCs may
traffic through tissues, pick up apoptotic cells arising from normal cell turnover,
and then, upon migration to lymph nodes in afferent lymph, silence T cells to self-antigens
in the phagocytosed apoptotic bodies. Tolerance to self-antigens in the steady state
need not be direct. It may instead involve transport of apoptotic bodies in short-lived
migratory DCs to longer-lived, tolerizing DCs in the lymph node. The latter are able
in the steady state to form high levels of MHC–peptide complexes but either lack key
costimulators for immunity or have unique products for inducing tolerance.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs.
M Albert, B Sauter, N Bhardwaj (1998)
- Record: found
- Abstract: found
- Article: not found
Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation
M. Cella, A Lanzavecchia, K Lehmann … (1996)
- Record: found
- Abstract: found
- Article: not found
Natural adjuvants: endogenous activators of dendritic cells.
S. Gallucci, M. Lolkema, P Matzinger (1999)
