- Record: found
- Abstract: found
- Article: found
Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis
Read this article at
Abstract
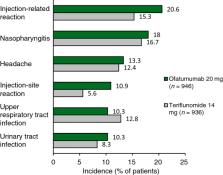
Ofatumumab (Kesimpta ®) is a fully human anti-CD20 monoclonal antibody that can be self-administered by patients and is approved in several countries worldwide for the treatment of relapsing forms of multiple sclerosis (MS). In two identical phase III trials in adults with relapsing forms of MS, subcutaneous ofatumumab was more effective than oral teriflunomide in reducing the annualized relapse rate, as well as reducing MRI-detected lesion activity and limiting worsening of disability. Ofatumumab had a generally manageable tolerability profile; the most common adverse events (AEs) included nasopharyngitis, headache, upper respiratory tract infections and urinary tract infections. AEs of special interest (AESIs) included infections and injection-related reactions, which were generally manageable. There was no apparent association between changes in immunoglobulin G or M levels and the risk of serious infections after 3.5 years of ofatumumab treatment. Thus, ofatumumab is a convenient treatment option that is effective and has a generally manageable tolerability profile in adults with relapsing forms of MS.
Plain Language Summary
MS is an incurable disease that affects ≈ 2.8 million people worldwide. Limiting the progression of disability associated with this disease is crucial, and treatments such as teriflunomide or monoclonal antibodies can prevent the relapses which define MS. Ofatumumab (Kesimpta ®), a monoclonal antibody, works by reducing the level of B cells which contribute to the development and progression of MS. Ofatumumab is approved in several countries worldwide to treat adults with certain relapsing forms of MS. It is administered by subcutaneous injection once per month and is the first therapy of its kind that patients can self-inject at home. In clinical trials, ofatumumab was more effective than teriflunomide in reducing the annual relapse rate, as well as slowing both the progression of disability and formation of new MS lesions in the brain. Ofatumumab had a generally manageable tolerability profile, although treatment resulted in infections and injection-related reactions; these were generally manageable with treatment. Thus, ofatumumab is an effective and convenient treatment option, with a generally manageable tolerability profile, in adults with relapsing forms of MS.
Related collections
Most cited references14

- Record: found
- Abstract: found
- Article: found
Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition
- Record: found
- Abstract: found
- Article: not found
Ofatumumab versus Teriflunomide in Multiple Sclerosis
- Record: found
- Abstract: found
- Article: not found