- Record: found
- Abstract: found
- Article: found
KRAS‐G12D mutation drives immune suppression and the primary resistance of anti‐PD‐1/PD‐L1 immunotherapy in non‐small cell lung cancer
Read this article at
Abstract
Background
Although immune checkpoint inhibitors (ICIs) against programmed cell death protein 1 (PD‐1) and its ligand PD‐L1 have demonstrated potency towards treating patients with non‐small cell lung carcinoma (NSCLC), the potential association between Kirsten rat sarcoma viral oncogene homolog ( KRAS) oncogene substitutions and the efficacy of ICIs remains unclear. In this study, we aimed to find point mutations in the KRAS gene resistant to ICIs and elucidate resistance mechanism.
Methods
The association between KRAS variant status and the efficacy of ICIs was explored with a clinical cohort ( n = 74), and confirmed with a mouse model. In addition, the tumor immune microenvironment (TIME) of KRAS‐mutant NSCLC, such as CD8 + tumor‐infiltrating lymphocytes (TILs) and PD‐L1 level, was investigated. Cell lines expressing classic KRAS substitutions were used to explore signaling pathway activation involved in the formation of TIME. Furthermore, interventions that improved TIME were developed to increase responsiveness to ICIs.
Results
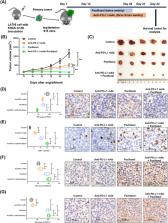
We observed the inferior efficacy of ICIs in KRAS‐G12D‐mutant NSCLC. Based upon transcriptome data and immunostaining results from KRAS‐mutant NSCLC, KRAS‐G12D point mutation negatively correlated with PD‐L1 level and secretion of chemokines CXCL10/CXCL11 that led to a decrease in CD8 + TILs, which in turn yielded an immunosuppressive TIME. The analysis of cell lines overexpressing classic KRAS substitutions further revealed that KRAS‐G12D mutation suppressed PD‐L1 level via the P70S6K/PI3K/AKT axis and reduced CXCL10/CXCL11 levels by down‐regulating high mobility group protein A2 (HMGA2) level. Notably, paclitaxel, a chemotherapeutic agent, upregulated HMGA2 level, and in turn, stimulated the secretion of CXCL10/CXCL11. Moreover, PD‐L1 blockade combined with paclitaxel significantly suppressed tumor growth compared with PD‐L1 inhibitor monotherapy in a mouse model with KRAS‐G12D‐mutant lung adenocarcinoma. Further analyses revealed that the combined treatment significantly enhanced the recruitment of CD8 + TILs via the up‐regulation of CXCL10/CXCL11 levels. Results of clinical study also revealed the superior efficacy of chemo‐immunotherapy in patients with KRAS‐G12D‐mutant NSCLC compared with ICI monotherapy.
Related collections
Most cited references67

- Record: found
- Abstract: found
- Article: found
Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2019
- Record: found
- Abstract: found
- Article: not found