- Record: found
- Abstract: found
- Article: found
Femtosecond pulsed laser photodynamic therapy activates melanin and eradicates malignant melanoma
Read this article at
Significance
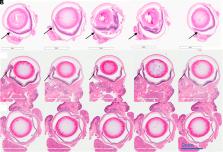
While effective in non-pigmented tumors, photodynamic therapy (PDT) has failed in treating melanoma due to the high light attenuation by melanin. Here, an effective approach for ocular melanoma is proposed using the concept of melanin-mediated multi-photon PDT. Experiments were performed comparing cytotoxicity in pigmented versus nonpigmented melanoma cells treated by 1- versus 2-photon PDT using low versus high 2-photon cross-section photosensitizers. The unexpected results show that, under femtosecond pulsed laser irradiation, melanin can absorb 2 and 3 photons and transfer the energy to the photosensitizer. The resulting therapeutic efficacy is demonstrated in vivo in a murine model of conjunctival melanoma, achieving complete tumor eradication and showing the potential of this approach as a minimally invasive therapeutic option.
Abstract
Photodynamic therapy (PDT) relies on a series of photophysical and photochemical reactions leading to cell death. While effective for various cancers, PDT has been less successful in treating pigmented melanoma due to high light absorption by melanin. Here, this limitation is addressed by 2-photon excitation of the photosensitizer (2p-PDT) using ~100 fs pulses of near-infrared laser light. A critical role of melanin in enabling rather than hindering 2p-PDT is elucidated using pigmented and non-pigmented murine melanoma clonal cell lines in vitro. The photocytotoxicities were compared between a clinical photosensitizer (Visudyne) and a porphyrin dimer (Oxdime) with ~600-fold higher σ 2p value. Unexpectedly, while the 1p-PDT responses are similar in both cell lines, 2p activation is much more effective in killing pigmented than non-pigmented cells, suggesting a dominant role of melanin 2p-PDT. The potential for clinical translational is demonstrated in a conjunctival melanoma model in vivo, where complete eradication of small tumors was achieved. This work elucidates the melanin contribution in multi-photon PDT enabling significant advancement of light-based treatments that have previously been considered unsuitable in pigmented tumors.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Uveal melanoma: trends in incidence, treatment, and survival.

- Record: found
- Abstract: found
- Article: found
Treatment of uveal melanoma: where are we now?
- Record: found
- Abstract: found
- Article: not found