- Record: found
- Abstract: found
- Article: found
Cellular senescence in osteoarthritis pathology
Read this article at
Summary
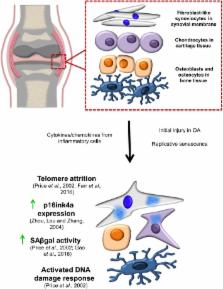
Cellular senescence is a state of stable proliferation arrest of cells. The senescence pathway has many beneficial effects and is seen to be activated in damaged/stressed cells, as well as during embryonic development and wound healing. However, the persistence and accumulation of senescent cells in various tissues can also impair function and have been implicated in the pathogenesis of many age‐related diseases. Osteoarthritis ( OA), a severely debilitating chronic condition characterized by progressive tissue remodeling and loss of joint function, is the most prevalent disease of the synovial joints, and increasing age is the primary OA risk factor. The profile of inflammatory and catabolic mediators present during the pathogenesis of OA is strikingly similar to the secretory profile observed in ‘classical’ senescent cells. During OA, chondrocytes (the sole cell type present within articular cartilage) exhibit increased levels of various senescence markers, such as senescence‐associated beta‐galactosidase ( SAβGal) activity, telomere attrition, and accumulation of p16ink4a. This suggests the hypothesis that senescence of cells within joint tissues may play a pathological role in the causation of OA. In this review, we discuss the mechanisms by which senescent cells may predispose synovial joints to the development and/or progression of OA, as well as touching upon various epigenetic alterations associated with both OA and senescence.
Related collections
Most cited references69

- Record: found
- Abstract: found
- Article: found
The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs
- Record: found
- Abstract: found
- Article: not found
Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion
- Record: found
- Abstract: found
- Article: not found