- Record: found
- Abstract: found
- Article: found
Harnessing the potential of CAR-T cell therapy: progress, challenges, and future directions in hematological and solid tumor treatments
Read this article at
Abstract
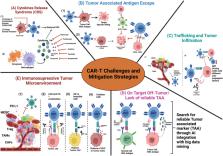
Traditional cancer treatments use nonspecific drugs and monoclonal antibodies to target tumor cells. Chimeric antigen receptor (CAR)-T cell therapy, however, leverages the immune system's T-cells to recognize and attack tumor cells. T-cells are isolated from patients and modified to target tumor-associated antigens. CAR-T therapy has achieved FDA approval for treating blood cancers like B-cell acute lymphoblastic leukemia, large B-cell lymphoma, and multiple myeloma by targeting CD-19 and B-cell maturation antigens. Bi-specific chimeric antigen receptors may contribute to mitigating tumor antigen escape, but their efficacy could be limited in cases where certain tumor cells do not express the targeted antigens. Despite success in blood cancers, CAR-T technology faces challenges in solid tumors, including lack of reliable tumor-associated antigens, hypoxic cores, immunosuppressive tumor environments, enhanced reactive oxygen species, and decreased T-cell infiltration. To overcome these challenges, current research aims to identify reliable tumor-associated antigens and develop cost-effective, tumor microenvironment-specific CAR-T cells. This review covers the evolution of CAR-T therapy against various tumors, including hematological and solid tumors, highlights challenges faced by CAR-T cell therapy, and suggests strategies to overcome these obstacles, such as utilizing single-cell RNA sequencing and artificial intelligence to optimize clinical-grade CAR-T cells.
Related collections
Most cited references388
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found
The blockade of immune checkpoints in cancer immunotherapy.
- Record: found
- Abstract: found
- Article: not found