- Record: found
- Abstract: found
- Article: found
Multifaceted Rho GTPase Signaling at the Endomembranes
Read this article at
Abstract
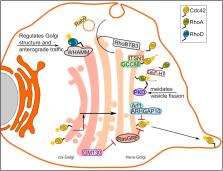
The Rho family of small GTPases orchestrates fundamental biological processes such as cell cycle progression, cell migration, and actin cytoskeleton dynamics, and their aberrant signaling is linked to numerous human diseases and disorders. Traditionally, active Rho GTPase proteins were proposed to reside and function predominantly at the plasma membrane. While this view still holds true, it is emerging that active pool of multiple Rho GTPases are in part localized to endomembranes such as endosomes and the Golgi. In this review, we will focus on the intracellular pools and discuss how their local activation contributes to the shaping of various cellular processes. Our main focus will be on Rho signaling from the endosomes, Golgi, mitochondria and nucleus and how they regulate multiple cellular events such as receptor trafficking, cell proliferation and differentiation, cell migration and polarity.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors.
- Record: found
- Abstract: found
- Article: not found
The 'invisible hand': regulation of RHO GTPases by RHOGDIs.
- Record: found
- Abstract: found
- Article: not found