- Record: found
- Abstract: found
- Article: found
The hypoxia–reoxygenation stress in plants

Read this article at
Abstract
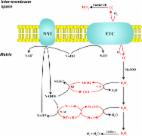
Plants are very plastic in adapting growth and development to changing adverse environmental conditions. This feature will be essential for plants to survive climate changes characterized by extreme temperatures and rainfall. Although plants require molecular oxygen (O 2) to live, they can overcome transient low-O 2 conditions (hypoxia) until return to standard 21% O 2 atmospheric conditions (normoxia). After heavy rainfall, submerged plants in flooded lands undergo transient hypoxia until water recedes and normoxia is recovered. The accumulated information on the physiological and molecular events occurring during the hypoxia phase contrasts with the limited knowledge on the reoxygenation process after hypoxia, which has often been overlooked in many studies in plants. Phenotypic alterations during recovery are due to potentiated oxidative stress generated by simultaneous reoxygenation and reillumination leading to cell damage. Besides processes such as N-degron proteolytic pathway-mediated O 2 sensing, or mitochondria-driven metabolic alterations, other molecular events controlling gene expression have been recently proposed as key regulators of hypoxia and reoxygenation. RNA regulatory functions, chromatin remodeling, protein synthesis, and post-translational modifications must all be studied in depth in the coming years to improve our knowledge on hypoxia–reoxygenation transition in plants, a topic with relevance in agricultural biotechnology in the context of global climate change.
Abstract
Plant responses to hypoxia and subsequent reoxygenation occur through a network of processes involving oxygen sensing, RNA function, chromatin remodeling, gene expression, and protein synthesis, which together allow plants to either escape or tolerate the stress.
Related collections
Most cited references187
- Record: found
- Abstract: found
- Article: found
Reperfusion injury and reactive oxygen species: The evolution of a concept☆
- Record: found
- Abstract: found
- Article: not found
Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization.
- Record: found
- Abstract: found
- Article: not found